- #1
ussername
- 60
- 2
Let's consider an isotherm isobaric adsorption of gas (A) on the adsorbent (B). There are two phases in the system:
- volume phase (1) that consists of gas and adsorbent.
- surface phase (2) that contains a layer of adsorbed gas on the surface of adsorbent.
When deriving Gibbs adsorption isotherm, the work needed to change the surface of interface of phase (1) is neglected, i.e. the Gibbs free energy changes for both phases are:
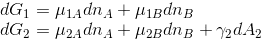
I wonder why one can neglect
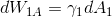 ? During adsorption the gas passes from phase (1) into phase (2) and the surface of interface of (1) is changing. Why is that?
? During adsorption the gas passes from phase (1) into phase (2) and the surface of interface of (1) is changing. Why is that?
- volume phase (1) that consists of gas and adsorbent.
- surface phase (2) that contains a layer of adsorbed gas on the surface of adsorbent.
When deriving Gibbs adsorption isotherm, the work needed to change the surface of interface of phase (1) is neglected, i.e. the Gibbs free energy changes for both phases are:
I wonder why one can neglect
Last edited by a moderator: