Callmelucky
- 144
- 30
- Homework Statement
- How to calculate work in cycle process that consists of isothermal, isobaric and adiabatic processes.
- Relevant Equations
- deltaU=Q-W, Q=W, Q=-W,
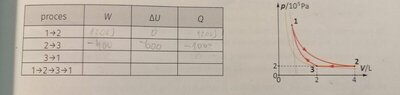
(picture of diagram below)So the task goes like this: gas is ideal. Process 3->1 s adiabatic and in process 1->2 work done is 1200J. Fill the table.
I don't know how to calculate work done in an adiabatic process because p2 and V2 are not given and I don't know gama(Cp/Cv).
I know that deltaU of the entire process is 0. So if I am thinking correctly work done should be 800J because work done from 2->3 is -400J(first two processes are correct) and that is energy gained back, so to reach 1200J of work we need 800J more, but it's not. Apparently work done in 3->1 is -600J, deltaU= 600J and Q=0. And total work done is 200J, deltaU=0, and Q= 200J.
Please help me.
I don't know how to calculate work done in an adiabatic process because p2 and V2 are not given and I don't know gama(Cp/Cv).
I know that deltaU of the entire process is 0. So if I am thinking correctly work done should be 800J because work done from 2->3 is -400J(first two processes are correct) and that is energy gained back, so to reach 1200J of work we need 800J more, but it's not. Apparently work done in 3->1 is -600J, deltaU= 600J and Q=0. And total work done is 200J, deltaU=0, and Q= 200J.
Please help me.