- #1
MG5
- 60
- 0
I need to write the lewis dot structure along with the 3 resonance forms for CH3CNS. This is what I had but it was wrong. Not sure what to do. Thanks.
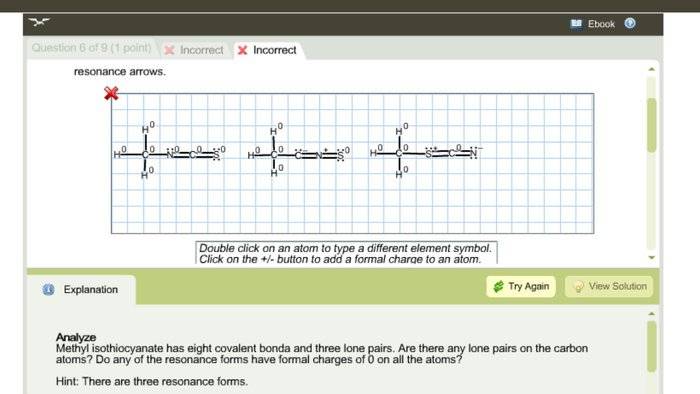
A Lewis structure is a representation of the valence electrons and bonds in a molecule. It consists of the element symbols and dots or lines to represent electrons.
To draw the Lewis structure for CH3NCS, first determine the total number of valence electrons by adding the valence electrons of each element: 4 for carbon, 1 for hydrogen, 5 for nitrogen, and 6 for sulfur. Then, arrange the atoms to show the correct connectivity, with carbon in the center and the other atoms surrounding it. Place the remaining electrons around the atoms to satisfy the octet rule, and use multiple bonds if necessary.
Resonance forms show the different ways in which electrons can be delocalized or shared among atoms in a molecule. This helps to explain the stability and reactivity of the molecule.
The most stable resonance form is the one with the lowest formal charges on the atoms and the most complete octets. In CH3NCS, the most stable resonance form is the one with a triple bond between carbon and nitrogen.
CH3NCS is a polar molecule because it has a slightly positive charge on the carbon atom and a slightly negative charge on the nitrogen atom due to the difference in electronegativity between these atoms. This creates a dipole moment, making the molecule polar.