- #1
etotheipi
I was doing a question that wanted you to determine the structure given a molecular formula and an NMR spectrum. The following was the answer:
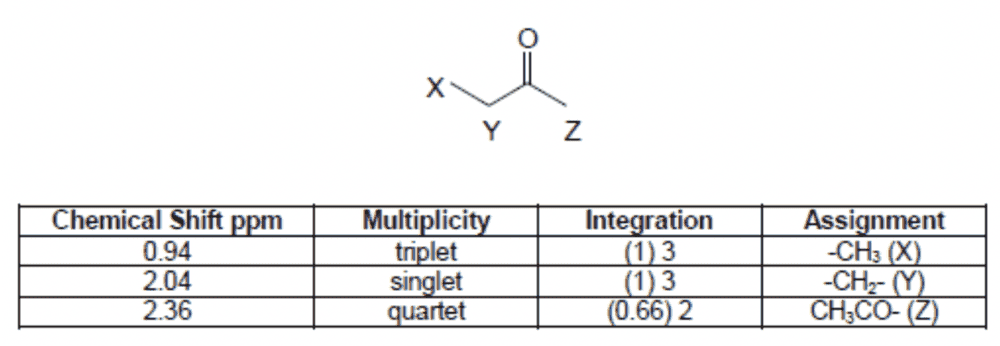
I'm unsure as to how the multiplicities were obtained. For ##X##, the neighbouring carbon ##Y## has 2 hydrogens, so this peak is a triplet (OK so far!).
But for ##Y##, the neighbouring carbon (##X##?) has 3 hydrogens so shouldn't that peak should be a quartet? And ##Z##'s neighbouring carbon has no hydrogens so this peak should just be a singlet. This also agrees with the integration, since both ##X## and ##Z## have 3 hydrogens but ##Y## only has 2. I am not sure why they have put it the other way around?
Thank you!
I'm unsure as to how the multiplicities were obtained. For ##X##, the neighbouring carbon ##Y## has 2 hydrogens, so this peak is a triplet (OK so far!).
But for ##Y##, the neighbouring carbon (##X##?) has 3 hydrogens so shouldn't that peak should be a quartet? And ##Z##'s neighbouring carbon has no hydrogens so this peak should just be a singlet. This also agrees with the integration, since both ##X## and ##Z## have 3 hydrogens but ##Y## only has 2. I am not sure why they have put it the other way around?
Thank you!