Devin-M
- 1,069
- 765
I am trying to reconcile HFC-134a refrigerant with the 2nd Law of Thermodynamics. I shouldn't be able to extract useful work from a single temperature reservoir.
Suppose I use a refrigeration / heat pump cycle with HFC-134a as shown below.
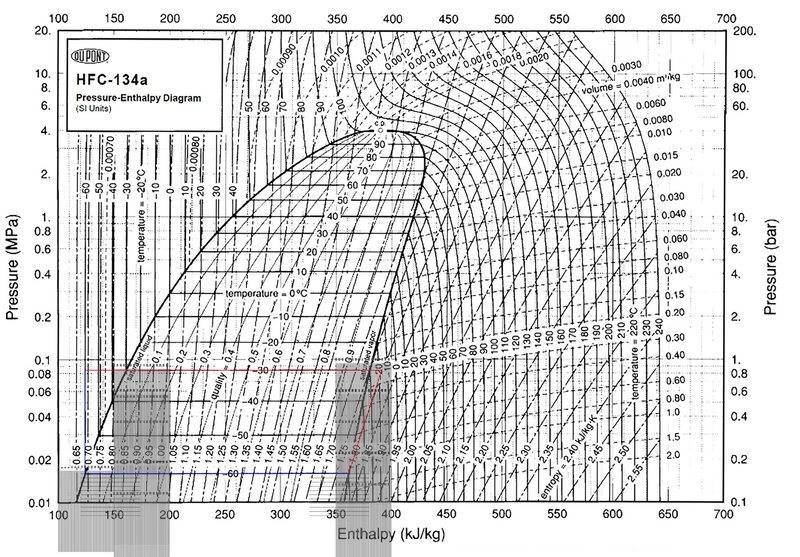
-Compressor adds 29 kJ/kg increasing refrigerant temperature from -60C (saturated vapor) to -18C
-Hot side heat exchanger is divided into 3 sections -- two variable temperature sections and one constant temperature section.
-The log mean temp of hot side section 1 is -24C, temp of hot side section 2 is -30C, and log mean temp of hot side section 3 is -45.3C
-If 3 carnot efficiency heat engines are connected across the 3 hot sections to the -60C cold section, they extract 30.96kJ/kg of useful work.
Since the compressor only adds 29kJ/kg of energy, but the useful work of the heat engines is 30.96kJ/kg, won't this mean there is 1.96kJ/kg energy deficit on the cold side of the loop before the compressor? Can't this cold section absorb heat from an outside heat reservoir that is greater than -60C to complete the cycle?
https://www.speakev.com/cdn-cgi/image/format=auto,onerror=redirect,width=1920,height=1920,fit=scale-down/https://www.speakev.com/attachments/hfc-134a6-jpg.147707/
Suppose I use a refrigeration / heat pump cycle with HFC-134a as shown below.
-Compressor adds 29 kJ/kg increasing refrigerant temperature from -60C (saturated vapor) to -18C
-Hot side heat exchanger is divided into 3 sections -- two variable temperature sections and one constant temperature section.
-The log mean temp of hot side section 1 is -24C, temp of hot side section 2 is -30C, and log mean temp of hot side section 3 is -45.3C
-If 3 carnot efficiency heat engines are connected across the 3 hot sections to the -60C cold section, they extract 30.96kJ/kg of useful work.
Since the compressor only adds 29kJ/kg of energy, but the useful work of the heat engines is 30.96kJ/kg, won't this mean there is 1.96kJ/kg energy deficit on the cold side of the loop before the compressor? Can't this cold section absorb heat from an outside heat reservoir that is greater than -60C to complete the cycle?
https://www.speakev.com/cdn-cgi/image/format=auto,onerror=redirect,width=1920,height=1920,fit=scale-down/https://www.speakev.com/attachments/hfc-134a6-jpg.147707/
Last edited: