cmcpeek
- 1
- 0
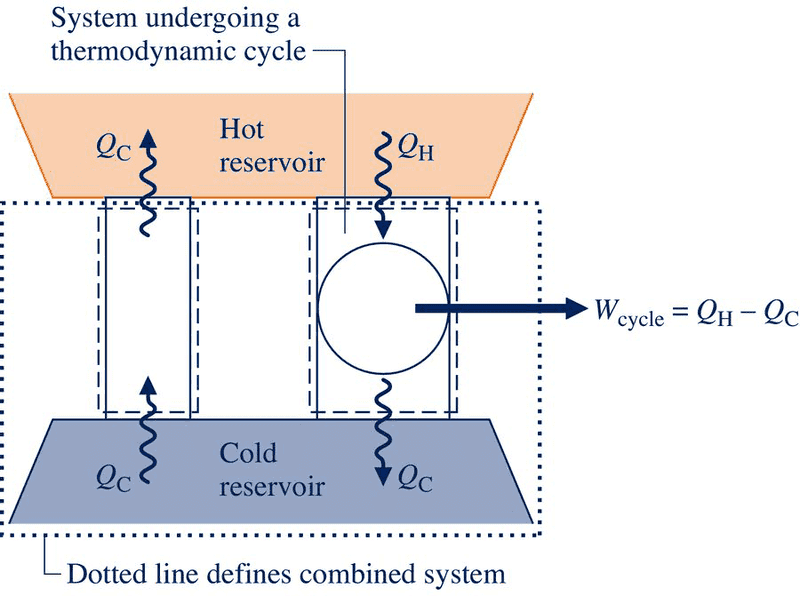
I have a quick question concerning the 2nd law of thermodynamics. So my textbook uses an illustration to explain how the violation of the Clausius statement of the 2nd law implies a violation of the Kelvin-Plank. But while looking over the diagram I stated thinking about a certain situation. In the diagram two systems are shown communicating thermally with the same reservoirs. The on on the left is in violation of the Clausius statement while the one on the right is thermodynamicaly sound as its own system. But when you draw the boundary line around the two cycles and the cold reservoir, it violates the Kelvin-Plank statement, which is perfectly okay. However, what if a system boundary were drawn around the cycle to the right and the cold reservoir, making a system consisting of those two entities. It would seem that the system would be communication thermally with only one reservoir (the hot one) yet generate a net output of work, which violates the Kelvin-Plank statement. Any insight would be appreciated.