- #1
prakhargupta3301
- 58
- 1
Phosphorus: sp3 hybridized
Fluorine (each one of three): sp3 hybridized
Back bonding can occur between only PURE orbitals ie those which are unhyridized.
Let's look at e- config. of both of them to be a little clear of what I'm asking:
P:
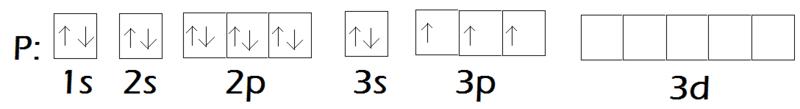
F:
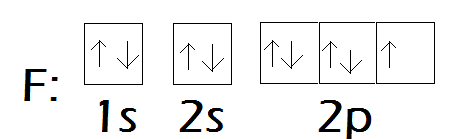
Since fluorine is sp3 hybridized, the orbitals ie either of px, py or pz (2 of which are filled and are lone pairs on F of the 3 total lone pairs) can't participate in back bonding with the unhiybridized d orbital of Phosphorus.
My question is, then how does it show back bonding? I know it does, but how?
Fluorine (each one of three): sp3 hybridized
Back bonding can occur between only PURE orbitals ie those which are unhyridized.
Let's look at e- config. of both of them to be a little clear of what I'm asking:
P:
F:
Since fluorine is sp3 hybridized, the orbitals ie either of px, py or pz (2 of which are filled and are lone pairs on F of the 3 total lone pairs) can't participate in back bonding with the unhiybridized d orbital of Phosphorus.
My question is, then how does it show back bonding? I know it does, but how?