Master1022
- 590
- 116
- Homework Statement
- For the reaction the following stoichiometric coefficients have been determined: 2 ##C## react to form 2 ##E## and 1 ##F##. The rate of formation of E is ##1.6 \times 10^{–4} mol L^{–1} s^{–1}##. What is the reaction rate and rate of usage of reactant C?
- Relevant Equations
- kinetics equations
Hi,
I was attempting the following question and don't know how to find the 'reaction rate':
"For the reaction the following stoichiometric coefficients have been determined: 2 ##C## react to form 2 ##E## and 1 ##F##. The rate of formation of E is ##1.6\times10^{–4} mol L^{–1} s^{–1}##. What is the reaction rate and rate of usage of reactant C? "
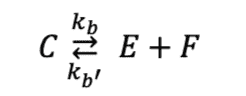
(note this form of the equation doesn't have the coefficients in front of it)
Attempt:
We can write the equations for the formation of E and C as follows:
\frac{de}{dt} = k_b c^2 - k_{b'} e^2 f = 1.6\times10^{–4} mol L^{–1} s^{–1}
\frac{dc}{dt} = k_{b'} e^2 f - k_b c^2
The second expression is just -1 times the first expression and I am assuming the: rate of formation = -1 * rate of usage. That all leads to the rate of usage of reactant C is ##1.6 \times10^{–4} mol L^{–1} s^{–1}##.
Now I am confused on how to find the reaction rate. The answer says it should be ##0.8\times10^{–4} mol L^{–1} s^{–1}## which I can see is a factor of 2 (or 0.5) away from our current rate, but I don't know how to connect the two. A search on google shows that:
\text{reaction rate} = \frac{-\Delta \text{[reactants]}}{\Delta t}
but it isn't clear how I use that expression to get the required answer.
Thanks for any help
I was attempting the following question and don't know how to find the 'reaction rate':
"For the reaction the following stoichiometric coefficients have been determined: 2 ##C## react to form 2 ##E## and 1 ##F##. The rate of formation of E is ##1.6\times10^{–4} mol L^{–1} s^{–1}##. What is the reaction rate and rate of usage of reactant C? "
(note this form of the equation doesn't have the coefficients in front of it)
Attempt:
We can write the equations for the formation of E and C as follows:
\frac{de}{dt} = k_b c^2 - k_{b'} e^2 f = 1.6\times10^{–4} mol L^{–1} s^{–1}
\frac{dc}{dt} = k_{b'} e^2 f - k_b c^2
The second expression is just -1 times the first expression and I am assuming the: rate of formation = -1 * rate of usage. That all leads to the rate of usage of reactant C is ##1.6 \times10^{–4} mol L^{–1} s^{–1}##.
Now I am confused on how to find the reaction rate. The answer says it should be ##0.8\times10^{–4} mol L^{–1} s^{–1}## which I can see is a factor of 2 (or 0.5) away from our current rate, but I don't know how to connect the two. A search on google shows that:
\text{reaction rate} = \frac{-\Delta \text{[reactants]}}{\Delta t}
but it isn't clear how I use that expression to get the required answer.
Thanks for any help