cheetah
- 2
- 0
- Homework Statement
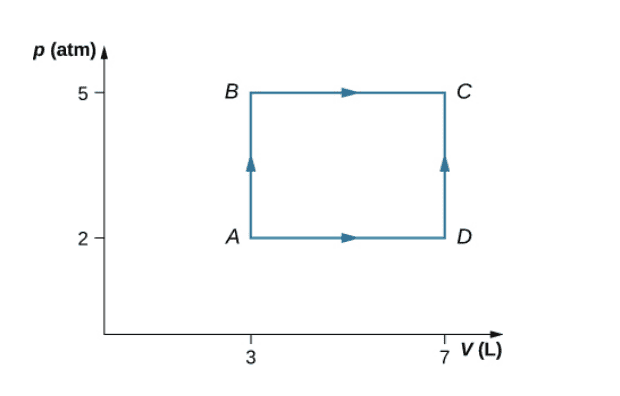
- Consider the processes shown in Fig. 1 for a monatomic gas.
a) Find the work done in each of the processes AB, BC, AD, and DC.
b) Find the internal energy change in processes AB and BC.
c) Find the internal energy difference between states C and A.
d) Find the total heat added in the ADC process.
e) From the information given, can you find the heat added in process AD? Why or why
not?
- Relevant Equations
- Eint = Q − W
I’m having trouble with a Thermodynamics Assignment and could use some help. I’ve been given the below graph and told to consider the processes shown for a monatomic gas. I’ve been asked to answer these questions with no further information besides the graph.