- #1
tinir
- 5
- 0
Many times, I find it hard to draw correct Lewis structure of molecules with nitrogen. For instance: the Lewis structure of H2CNN. I thought it should be double bond between C and N and single bond between N and N, like this:
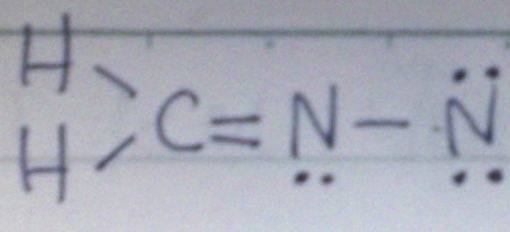
But actually it's not. I have done many problems like this wrong.
Please help me out! Thx!
But actually it's not. I have done many problems like this wrong.
Please help me out! Thx!