- #1
Saracen Rue
- 150
- 10
Hi, I'm currently doing an assignment on nuclear physics. One of the questions in said assignment is asking me to state the decays which usually happen within a nuclear power plant, as well as stating the fission products, explaining what's happening, and showing the equation. One of the sites my teacher linked me to for help with the assignment was this one: http://www.world-nuclear.org/Nuclear-Basics/How-does-a-nuclear-reactor-make-electricity-/. On the page there is an image which has confused me somewhat.
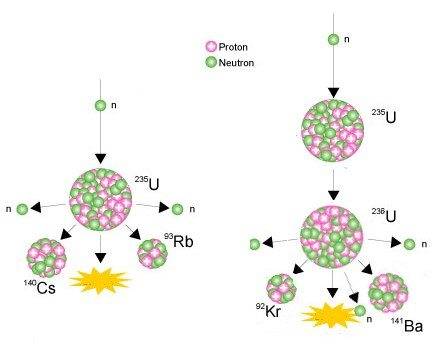
I understand the process happening on the right-hand side fine. A neutron being fired into the nucleus of U-235, creating U-236. Due to U-236 being unstable, it decays into Ba-141, Kr-92, 3 neutrons and releases energy. However, the process on the left-hand side has me stumped. From what I understand, the U-235 is getting a neutron fired into it, but instead of turning into U-236, it decays straight away. At first I thought they may have just skipped a step and we're meant to assume that it turning into U-236, but this is not the case as the masses of the fission products only add up to 235.
So, I guess my questions are a) How is it possible for U-235 to get a neutron fired at it and decay straight away, without ever becoming U-236 and b) What has happened to the neutron that was original fired into the nucleus?
Any help regarding this matter is greatly appreciated.
I understand the process happening on the right-hand side fine. A neutron being fired into the nucleus of U-235, creating U-236. Due to U-236 being unstable, it decays into Ba-141, Kr-92, 3 neutrons and releases energy. However, the process on the left-hand side has me stumped. From what I understand, the U-235 is getting a neutron fired into it, but instead of turning into U-236, it decays straight away. At first I thought they may have just skipped a step and we're meant to assume that it turning into U-236, but this is not the case as the masses of the fission products only add up to 235.
So, I guess my questions are a) How is it possible for U-235 to get a neutron fired at it and decay straight away, without ever becoming U-236 and b) What has happened to the neutron that was original fired into the nucleus?
Any help regarding this matter is greatly appreciated.