Woopa
- 20
- 4
- Homework Statement
- A container of an ideal gas that is isolated from its surroundings is divided into two parts. One part has double the volume of the other. The pressure in each part is p and the temperature is the same. The partition is removed. What is the pressure in the container now?
- Relevant Equations
- PV=nRT
Question:
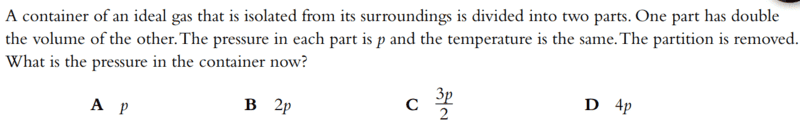
Answer:
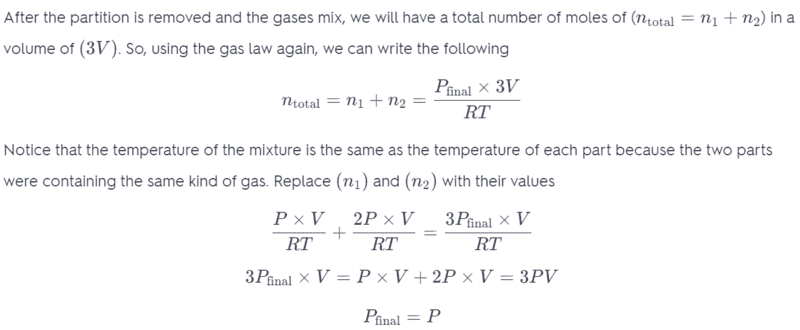
In the third last line of working, I do not understand why the pressure variable is changing? Shouldn't pressure remain constant and only the Volume change?
Answer:
In the third last line of working, I do not understand why the pressure variable is changing? Shouldn't pressure remain constant and only the Volume change?