Juanda
Gold Member
- 439
- 144
- TL;DR Summary
- A different thread was posted and I couldn't understand it. I was recommended to open a new thread to keep the original one on track.
Last month @Chestermiller opened the thread: Focus Problem for Entropy Change in Irreversible Adiabatic Process.
I couldn't wrap my head around something apparently simple but the thread was not about that so I was instructed to open a new thread to discuss it separately and keep the original thread on track.
I owned this thread to Chestermiller for a while now and finally, I have the chance to open it.
I'll copy and paste the same problem statement only changing the instant drop in external pressure with the piston being released from some braking system which is easier for me to visualize and I believe should have the same consequences.
I have an ideal gas in a cylinder with a massless, frictionless piston, and the gas starts out at To, Po, Vo. The system is adiabatic. The gas is in equilibrium at Po because the piston is held in place by a braking system. I initiate an irreversible process by releasing the brakes so the piston is exposed to the lower external pressure P1 which is constant while the gas expands and equilibrates with the new external pressure P1 at a new volume and temperate V1 and T1.
(a) Based on the 1st law of thermodynamics and the ideal gas law, what are the values of the new volume and temperature (in terms of Po, To, Vo, and P1)? How much irreversible work was done? What was the change in U?
(b) Describe at least 2 reversible processes you have devised to transition from the same initial state to the same final state. How much work was done, how much heat was added in each, and what was the change in U for these reversible processes?
(c). What was the change in entropy for the reversible processes? What was the change in entropy of the irreversible process?
So the problem has 3 questions (a, b, and c) although I'm already stuck in the first one. Mathematically I think I can find the state 1 (##P_1##, ##T_1## and ##V_1##) when the external and internal pressures are the same (##P_0## expands towards ##P_1##) using the adiabatic process relation because it's an adiabatic system so it should be an adiabatic expansion and the ideal gas law.
$$P_0V_0^\gamma=P_1V_1^\gamma \rightarrow V_1=e^\frac{\ln \frac{P_0V_0^\gamma}{P_1}}{\gamma} \tag{1}$$
$$P_1V_1=mRT_1 \rightarrow T_1 = \frac{P_1V_1}{mR} \tag{2}$$
But I don't see how the system can be in equilibrium when it reaches that point.
The way I see it, the work done by the adiabatic expansion of the gas inside the piston must be equal to the work done in the isobaric compression of the gas outside the piston because energy has no other place to go since heat transfer is not allowed (adiabatic system) and any other path for energy has been restricted (frictionless, massless, etc.).
The gas inside the piston will expand until its pressure levels with the outside pressure (P0→P1).
The energy required for such expansion would come from the internal energy of the gas inside the piston which can be expressed as a function of temperature (ideal gas).
However, I don't see how those two Works (adiabatic expansion in the piston and isobaric expansion outside) can be the same.
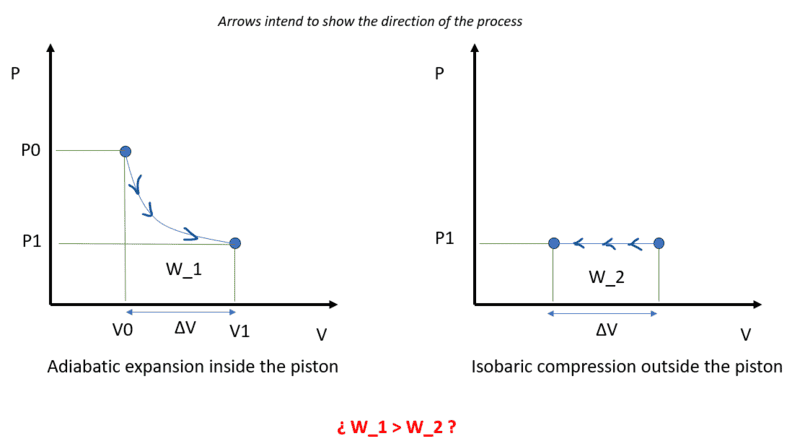
I'd need to include something else. For example, if the piston had mass, the difference in Works would be absorbed as kinetic energy of the piston and would be ##K_p = W_1-W_2##. In that case, the piston would reach the equilibrium point with non-zero velocity so it would overshoot and an oscillating movement would start. With friction being removed from the problem as the statement declares, the oscillation would keep going indefinitely.
This differs a lot when compared with what was done in the original thread. For example, Vanhees71 used the thermal energy in the gas to match the isobaric compression of the outside gas but I don't understand why it's possible to do that if it's been established that the piston undergoes an adiabatic expansion.
PS: On a little unrelated note. I have trouble understanding the units in equation ##(1)##. On the left side, we have units for volume ##[m^3]## as expected but on the right side, after doing some simplifications I arrive at ##[m^3]^\gamma##. Is that OK? I don't see how ##[m^3]=[m^3]^\gamma##. I am aware of \gamma being unitless but still has an impact on the equation.
I couldn't wrap my head around something apparently simple but the thread was not about that so I was instructed to open a new thread to discuss it separately and keep the original thread on track.
I owned this thread to Chestermiller for a while now and finally, I have the chance to open it.
I'll copy and paste the same problem statement only changing the instant drop in external pressure with the piston being released from some braking system which is easier for me to visualize and I believe should have the same consequences.
I have an ideal gas in a cylinder with a massless, frictionless piston, and the gas starts out at To, Po, Vo. The system is adiabatic. The gas is in equilibrium at Po because the piston is held in place by a braking system. I initiate an irreversible process by releasing the brakes so the piston is exposed to the lower external pressure P1 which is constant while the gas expands and equilibrates with the new external pressure P1 at a new volume and temperate V1 and T1.
(a) Based on the 1st law of thermodynamics and the ideal gas law, what are the values of the new volume and temperature (in terms of Po, To, Vo, and P1)? How much irreversible work was done? What was the change in U?
(b) Describe at least 2 reversible processes you have devised to transition from the same initial state to the same final state. How much work was done, how much heat was added in each, and what was the change in U for these reversible processes?
(c). What was the change in entropy for the reversible processes? What was the change in entropy of the irreversible process?
So the problem has 3 questions (a, b, and c) although I'm already stuck in the first one. Mathematically I think I can find the state 1 (##P_1##, ##T_1## and ##V_1##) when the external and internal pressures are the same (##P_0## expands towards ##P_1##) using the adiabatic process relation because it's an adiabatic system so it should be an adiabatic expansion and the ideal gas law.
$$P_0V_0^\gamma=P_1V_1^\gamma \rightarrow V_1=e^\frac{\ln \frac{P_0V_0^\gamma}{P_1}}{\gamma} \tag{1}$$
$$P_1V_1=mRT_1 \rightarrow T_1 = \frac{P_1V_1}{mR} \tag{2}$$
But I don't see how the system can be in equilibrium when it reaches that point.
The way I see it, the work done by the adiabatic expansion of the gas inside the piston must be equal to the work done in the isobaric compression of the gas outside the piston because energy has no other place to go since heat transfer is not allowed (adiabatic system) and any other path for energy has been restricted (frictionless, massless, etc.).
The gas inside the piston will expand until its pressure levels with the outside pressure (P0→P1).
The energy required for such expansion would come from the internal energy of the gas inside the piston which can be expressed as a function of temperature (ideal gas).
However, I don't see how those two Works (adiabatic expansion in the piston and isobaric expansion outside) can be the same.
I'd need to include something else. For example, if the piston had mass, the difference in Works would be absorbed as kinetic energy of the piston and would be ##K_p = W_1-W_2##. In that case, the piston would reach the equilibrium point with non-zero velocity so it would overshoot and an oscillating movement would start. With friction being removed from the problem as the statement declares, the oscillation would keep going indefinitely.
This differs a lot when compared with what was done in the original thread. For example, Vanhees71 used the thermal energy in the gas to match the isobaric compression of the outside gas but I don't understand why it's possible to do that if it's been established that the piston undergoes an adiabatic expansion.
PS: On a little unrelated note. I have trouble understanding the units in equation ##(1)##. On the left side, we have units for volume ##[m^3]## as expected but on the right side, after doing some simplifications I arrive at ##[m^3]^\gamma##. Is that OK? I don't see how ##[m^3]=[m^3]^\gamma##. I am aware of \gamma being unitless but still has an impact on the equation.
