- #1
pivoxa15
- 2,255
- 1
Is all chemistry ultimately about electrons in atoms and their configurations? If there is an electron reconfiguration then chemistry is involved a new chemical entity comes into existence.
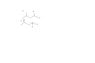
So take 2,4-dimethylpentane. It has two types which cannot be superimposed onto each other. One where both methyle groups are in the same direction. The other one where one methyl group is on top the other down the bottom.
However, one can be made into the other without an electron reconfiguration so these two molecules are not isomers of each other even though they are not superimposible?
So take 2,4-dimethylpentane. It has two types which cannot be superimposed onto each other. One where both methyle groups are in the same direction. The other one where one methyl group is on top the other down the bottom.
However, one can be made into the other without an electron reconfiguration so these two molecules are not isomers of each other even though they are not superimposible?