- #1
prakhargupta3301
- 58
- 1
Who am I kidding? Of course it is. But, everywhere I look, the series of increasing levels of orbitals is till
σ*2pz only
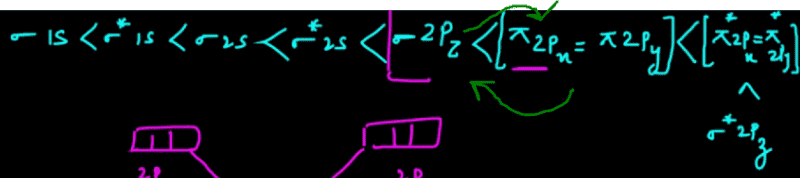
That's all.
So if I need to find bond order of , say, some molecule with greater number of e- like BF3 with 24 electrons, how do I proceed?
σ*2pz only
That's all.
So if I need to find bond order of , say, some molecule with greater number of e- like BF3 with 24 electrons, how do I proceed?