- #1
Pushoam
- 962
- 52
Homework Statement
Homework Equations
The Attempt at a Solution
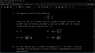
##C_p - C_v = p \frac{\partial V}{\partial T} ##
Doing the above, I got ## R\{ 1+ \frac{bp} {RT^2}\}##, there is no square in the right factor.
I want to know whether my answer is correct.