- #1
nezahualcoyot
- 5
- 1
The figure below is from a textbook. It is explaining what excited states are using carbon as an example. I don't necessarily agree that the the state labeled as "example excited state 1" is really an excited state. Since the electrons in the 2p orbitals are unpaired, and in the absence of a magnetic field spin up and spin down electrons have the same energy, I think this state has the same energy as the ground state.
My understanding of the Hund's rule is that electrons in degenerate energy orbitals are accommodated to maximize spin multiplicity, but again, these two states have the same spin multiplicity. Am I correct in my thinking that this is not really an excited state?
( Note in case the figure does not show correctly: The figure claims that an state with two unpaired electrons in the 2p orbitals for carbon is an excited state if the electrons have opposite spin. Each of these electrons is in a different 2p orbital. )
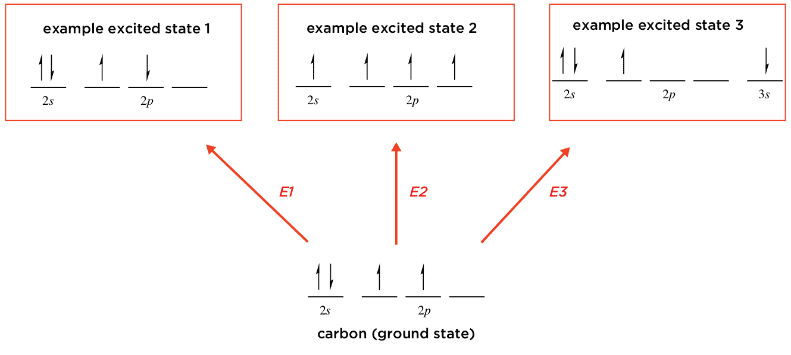
My understanding of the Hund's rule is that electrons in degenerate energy orbitals are accommodated to maximize spin multiplicity, but again, these two states have the same spin multiplicity. Am I correct in my thinking that this is not really an excited state?
( Note in case the figure does not show correctly: The figure claims that an state with two unpaired electrons in the 2p orbitals for carbon is an excited state if the electrons have opposite spin. Each of these electrons is in a different 2p orbital. )