- #1
theintarnets
- 64
- 0
Homework Statement
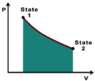
I am supposed to determine whether work is being done on or by a closed thermodynamic system from a diagram that's sort of like this, and provide justification for my answer:
My problem is I have no idea how to tell if that system is doing work or if work is being done on it based on the graph. I heard that if it's going in the clockwise direction, that means the work is being done by the system, but that isn't a proper justification. Can someone help me understand this better?