- #1
Psychae
- 6
- 0
I'm wondering if anyone can help me with the reasoning for what follows, I'm thinking I must just be missing something quite obvious but I can't seem to see what that is at the moment! 
I understand how to get the structure by starting from a 'skeleton structure' and just filling in electrons and then creating the double bond so that each atom has a complete octet (and to get FC as close to 0 and ignoring resonance structures etc):
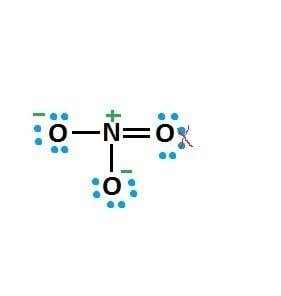
But I start to have difficulty when I try and do it showing all the valence electrons, I know the right structure:
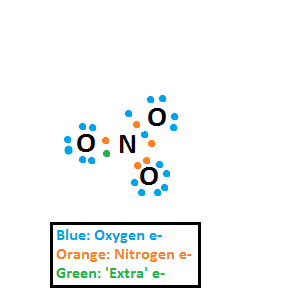 but I'm just wondering how how get that if you start drawing it yourself? I started by drawing all of the atoms with each of their electrons shown singly and then pairing them, and then adding in the 'extra' electron, so it's something like this:
but I'm just wondering how how get that if you start drawing it yourself? I started by drawing all of the atoms with each of their electrons shown singly and then pairing them, and then adding in the 'extra' electron, so it's something like this:
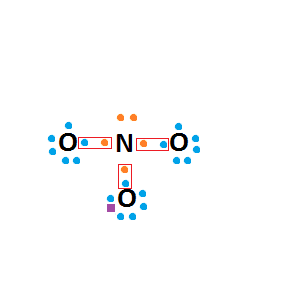
I'm just not sure how you work it out, and the coordinate bond seems to be making it even more difficult for me!
Sorry for the length of this, there were a couple of other things I was going to ask about it but I'm thinking they might be obvious if I get the answer to this so left them out. Any help would be greatly appreciated!

I understand how to get the structure by starting from a 'skeleton structure' and just filling in electrons and then creating the double bond so that each atom has a complete octet (and to get FC as close to 0 and ignoring resonance structures etc):
But I start to have difficulty when I try and do it showing all the valence electrons, I know the right structure:
I'm just not sure how you work it out, and the coordinate bond seems to be making it even more difficult for me!
Sorry for the length of this, there were a couple of other things I was going to ask about it but I'm thinking they might be obvious if I get the answer to this so left them out. Any help would be greatly appreciated!