- #1
HelloCthulhu
- 151
- 3
I recently read a paper on using an electric field to drive water autoionizaton. I'm trying to figure out how to use the Laplace equation on pg 9; 4th paragraph; to solve for voltage. I'm also interested in how this equation would change if I replaced the hemispherical tip with a parallel plate. Anyone strongly familiar with this subject matter? Any help is greatly appreciated!
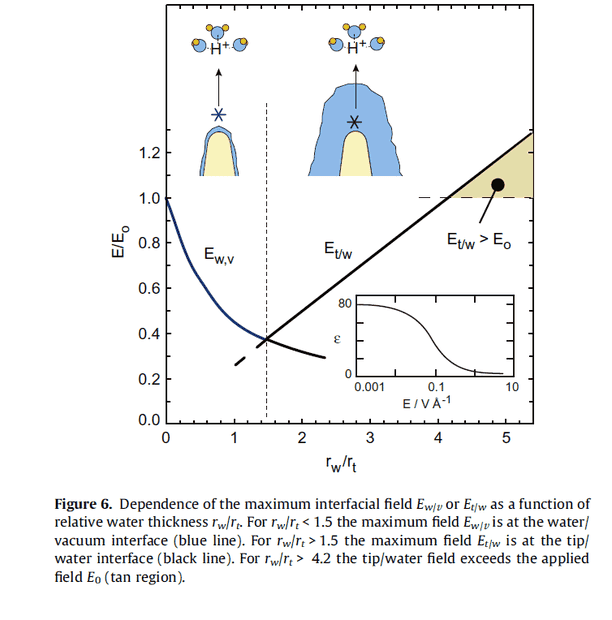
https://www.sciencedirect.com/science/article/abs/pii/S0009261411011511
https://www.sciencedirect.com/science/article/abs/pii/S0009261411011511