Sunwoo Bae
- 60
- 4
- Homework Statement
- Naming the given structures
- Relevant Equations
- None
When there is both alkyl substituent and ethoxy substituent in a single molecule, which one should be prioritized when numbering your base carbon chains?
I am confused because the first structure below seems to have numbered the carbon attached to ethyl as the first carbon, while the second structure seem to give carbon attached to ethoxy group the lowest carbon number.
Thanks for your help!
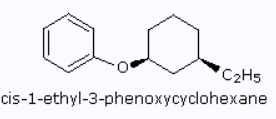
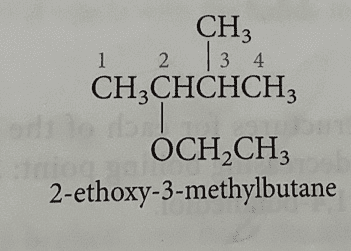
I am confused because the first structure below seems to have numbered the carbon attached to ethyl as the first carbon, while the second structure seem to give carbon attached to ethoxy group the lowest carbon number.
Thanks for your help!