Popz
- 5
- 0
- Homework Statement
- I have done most part of the data analysis. I am stuck with rearranging the equation in the correct way.
- Relevant Equations
- see below
Hey!
I am trying to determine the bond length of acetylene by analysing the vibrational spectrum of both H-Acetylene and D-Acetylene. I was able to calculate both the rotational constant and the moment of inertia but am stuck now as to how I get to the correct solution.
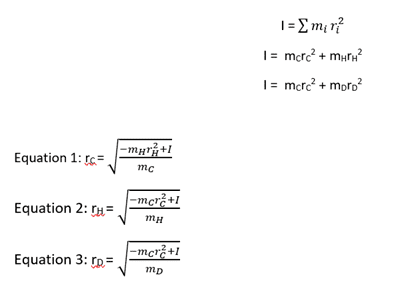
On the left is the original moment of inertia equation for polyatomic molecules which I have expanded to be specific for acetylene.
Then, on the right side are my rearrangements for the bond length of the respective molecules. I have been told that this is solvable by using two simultaneous equations - the moment of inertia for H and D. But I don't know how to solve this in practice.

However, I have been told that this is (probably) the correct equation. But I don't know how I get to this point.
I understand that the basic rearrangement equals r = √(I/2m) but, for example, I don't know why r(D) - r(H) would equal r(H) if that's what the first equation implies?
Hope that's not too confusing.
Thanks!
I am trying to determine the bond length of acetylene by analysing the vibrational spectrum of both H-Acetylene and D-Acetylene. I was able to calculate both the rotational constant and the moment of inertia but am stuck now as to how I get to the correct solution.
On the left is the original moment of inertia equation for polyatomic molecules which I have expanded to be specific for acetylene.
Then, on the right side are my rearrangements for the bond length of the respective molecules. I have been told that this is solvable by using two simultaneous equations - the moment of inertia for H and D. But I don't know how to solve this in practice.
However, I have been told that this is (probably) the correct equation. But I don't know how I get to this point.
I understand that the basic rearrangement equals r = √(I/2m) but, for example, I don't know why r(D) - r(H) would equal r(H) if that's what the first equation implies?
Hope that's not too confusing.
Thanks!