- #1
elemental09
- 42
- 0
I am participating in a reading course on nonlinear optics, which is a little difficult since I haven't had any formal education in quantum mechanics other than the standard introductory solving of Schroedinger's eqn. in 1D. Happily this course takes the semiclassical approach, in which the field is classical while all the atomic physics is treated quantum mechanically. The principle text I'm reading from is Nonlinear Optics, 3rd ed., by Boyd.
As an approximate starting point, (in Ch. 3 if you have the text), Boyd derives expressions for the first, second and third order susceptibility tensors. He takes two approaches: first, perturbatively solving the Schroedinger equation, and second, using the density matrix formalism. For both, the system considered is a single atom. In the first approach, it is assumed isolated. The density matrix approach then allows some consideration of the atom's environment. For both approaches he takes a Hamiltonian of the form
http://latex.codecogs.com/gif.latex?\hat{H} = \hat{H_{0}}+\hat{V}(t)
Where
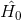 is the Hamiltonian of the free atom, and
is the Hamiltonian of the free atom, and
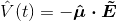
I have two questions to begin with, arising from my lack of background in QM:
1) Boyd refers to the wavefunction under consideration as the "atomic wavefunction". The wavefuntions for the eigenstates of the Hamiltonian then correspond to the quantized energy levels of the atom, but what other meaning is there to the wavefunction? For a single particle, I'm familiar with the probability amplitude for position measurement interpretation of the wavefunction, but this seemingly does not apply to this particular "atomic" wavefunction, as it is not the dynamics of the entire contiguous atom we are interested in, but rather the internal electronic behaviour (polarization and energy). Seemingly, then, we should be considering the wavefunctions of the individual electrons in the atom, though of course I understand that is analytically infeasible. That leads to my next question:
2) What is the meaning of
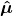 ? Boyd calls this the electric dipole moment operator. He defines it as
? Boyd calls this the electric dipole moment operator. He defines it as
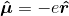 , where e is the charge on the electron. However, as discussed above, the states under consideration are atomic states. The individual electrons' wavefunctions are never described. I understand how this operator would be used to characterize the interaction energy of an electron with a classical field, but not an entire, neutral atom. Boyd goes on to compute the expectation value of the atom's electric dipole moment, which is given as:
, where e is the charge on the electron. However, as discussed above, the states under consideration are atomic states. The individual electrons' wavefunctions are never described. I understand how this operator would be used to characterize the interaction energy of an electron with a classical field, but not an entire, neutral atom. Boyd goes on to compute the expectation value of the atom's electric dipole moment, which is given as:

But this state is that for an atom, not an electron. Intuitively, it seems to me that a calculation of the expectation value of an electric dipole moment should involve the position operator acting on an electron's wavefunction, not an atom's.
I appreciate any effort to help me understand this. Sorry if these are standard textbook questions. If so, please feel free to direct me to a text at a good level (probably late undergrad or early grad QM) that would shed some light on all this.
As an approximate starting point, (in Ch. 3 if you have the text), Boyd derives expressions for the first, second and third order susceptibility tensors. He takes two approaches: first, perturbatively solving the Schroedinger equation, and second, using the density matrix formalism. For both, the system considered is a single atom. In the first approach, it is assumed isolated. The density matrix approach then allows some consideration of the atom's environment. For both approaches he takes a Hamiltonian of the form
http://latex.codecogs.com/gif.latex?\hat{H} = \hat{H_{0}}+\hat{V}(t)
Where
I have two questions to begin with, arising from my lack of background in QM:
1) Boyd refers to the wavefunction under consideration as the "atomic wavefunction". The wavefuntions for the eigenstates of the Hamiltonian then correspond to the quantized energy levels of the atom, but what other meaning is there to the wavefunction? For a single particle, I'm familiar with the probability amplitude for position measurement interpretation of the wavefunction, but this seemingly does not apply to this particular "atomic" wavefunction, as it is not the dynamics of the entire contiguous atom we are interested in, but rather the internal electronic behaviour (polarization and energy). Seemingly, then, we should be considering the wavefunctions of the individual electrons in the atom, though of course I understand that is analytically infeasible. That leads to my next question:
2) What is the meaning of
But this state is that for an atom, not an electron. Intuitively, it seems to me that a calculation of the expectation value of an electric dipole moment should involve the position operator acting on an electron's wavefunction, not an atom's.
I appreciate any effort to help me understand this. Sorry if these are standard textbook questions. If so, please feel free to direct me to a text at a good level (probably late undergrad or early grad QM) that would shed some light on all this.
Last edited by a moderator: