- #1
Taylor_1989
- 402
- 14
Hi guys, I am having an issue with what my lecture is saying in these slides, I have attched my slides below.
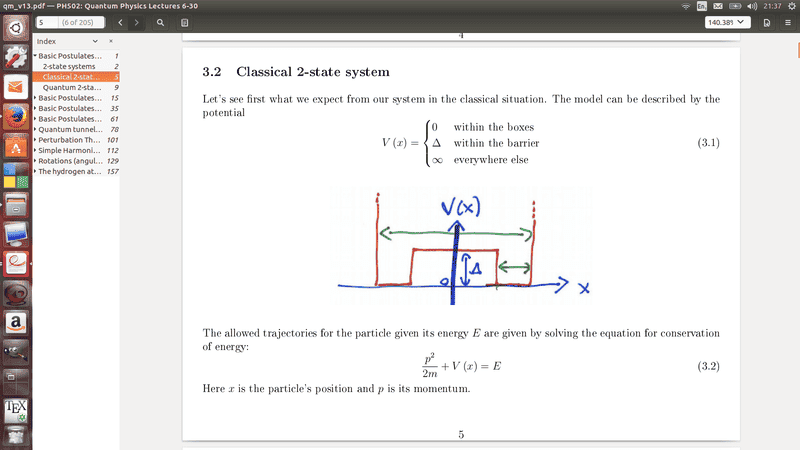
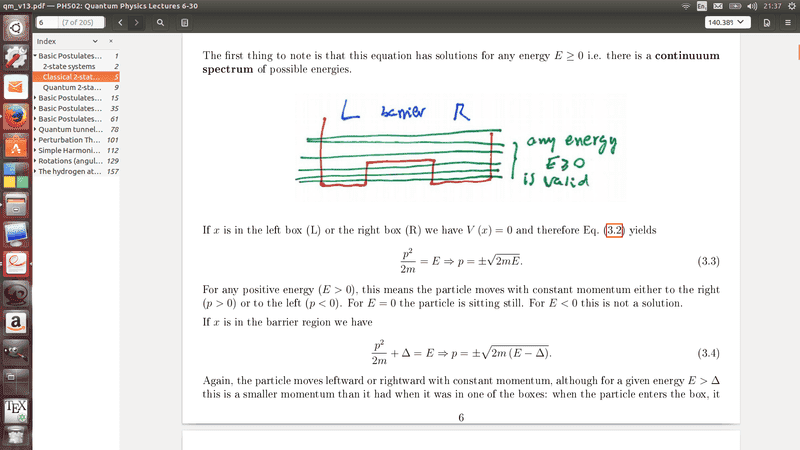
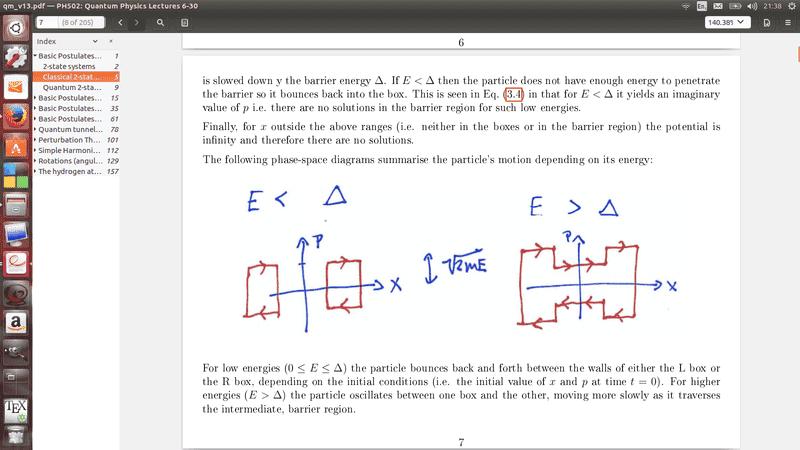
Here is my issue. I am very confused by the ##E<\Delta ## Beacuse I can't see how this has any momentum as it would produce an imagery number. And ye say that ##E<\Delta ## cannot be a solution yet on these diagrams he show the particle has momentum, at E=0 which can't be correct. I know I have this back to front, because he also mentions that at ##E>\Delta ## that is has less momentum than but I just can't see how, could someone please explain to me what my lecture is conveying here.
Here is my issue. I am very confused by the ##E<\Delta ## Beacuse I can't see how this has any momentum as it would produce an imagery number. And ye say that ##E<\Delta ## cannot be a solution yet on these diagrams he show the particle has momentum, at E=0 which can't be correct. I know I have this back to front, because he also mentions that at ##E>\Delta ## that is has less momentum than but I just can't see how, could someone please explain to me what my lecture is conveying here.





