- #1
patric44
- 308
- 40
- Homework Statement
- question about perturbation method treatment ?
- Relevant Equations
- in the pictures
hi guys
i am a the third year undergrad student and in this 2nd semester in my collage we should start taking quantum mechanics along with
molecular physics , our molecular physics professor choose a book that we are going to take which is " molecular physics by wolfgang Demtroder "
when i opened that book i found that it uses some advanced quantum mechanics like perturbation theory ... i found it really heavy for a student that just has some relativity intermediate quantum mechanics knowledge !
i started to get this perturbation theory thing ! but has some questions about this :
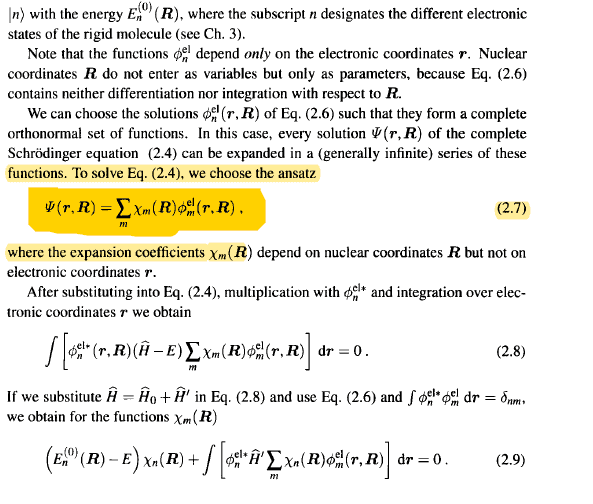
i get that he can represent the electronic distribution as a linear combination of functions with some coefficients that depend on the
position of the nuclei , how did he expand that integral and reached that partial derivative term its very unclear to me ?
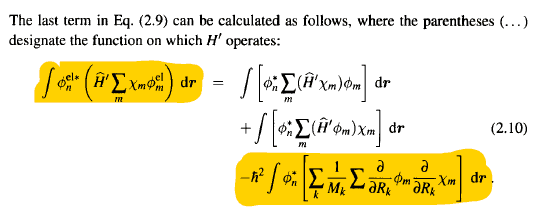
i appreciate any help
thanks
i am a the third year undergrad student and in this 2nd semester in my collage we should start taking quantum mechanics along with
molecular physics , our molecular physics professor choose a book that we are going to take which is " molecular physics by wolfgang Demtroder "
when i opened that book i found that it uses some advanced quantum mechanics like perturbation theory ... i found it really heavy for a student that just has some relativity intermediate quantum mechanics knowledge !
i started to get this perturbation theory thing ! but has some questions about this :
i get that he can represent the electronic distribution as a linear combination of functions with some coefficients that depend on the
position of the nuclei , how did he expand that integral and reached that partial derivative term its very unclear to me ?
i appreciate any help
thanks