- #1
Porkaborg
- 3
- 0
- TL;DR Summary
- Primitive unit cell of diamond, great confusion.
Hi guys , I want to construct a primitive unit cell for diamond, which is made of a fcc lattice and a basis of 2 carbons atoms. I know that a primitive unit cell isn't unique but the two variants I get are drastically different . As far as I can see they both include 2 whole atoms/points in the lattice, however one of them occupies 1/4 of the conventional-cell volume, and the other occupies 1/8 of the conventional-cell volume.
When I couldn't get further in reasoning, I tried googling it and found both versions listed as primitive unit cells for diamond. But I am still confused why they look so very different.
(The left picture doesn't include rest of the atoms for some reason but they are there before creating the primitive unit cell)
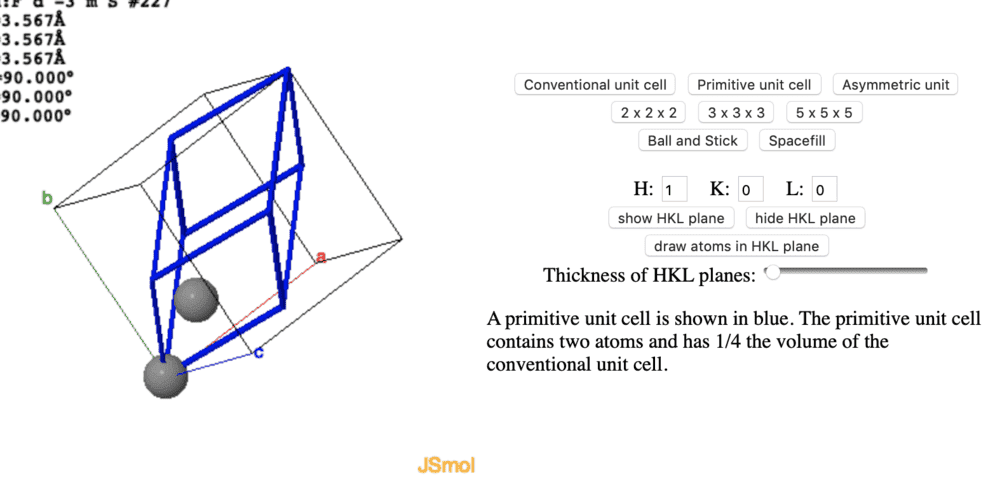
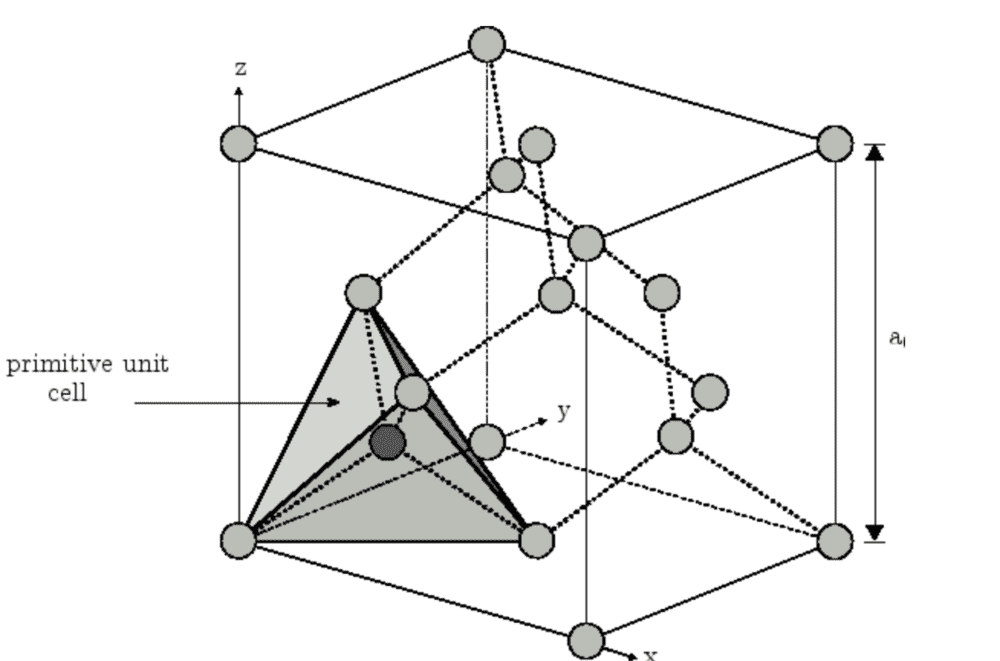
I hope someone can point out the difference , or pinpoint why I may be confused, please comment if I need to add some additional info.
When I couldn't get further in reasoning, I tried googling it and found both versions listed as primitive unit cells for diamond. But I am still confused why they look so very different.
(The left picture doesn't include rest of the atoms for some reason but they are there before creating the primitive unit cell)
I hope someone can point out the difference , or pinpoint why I may be confused, please comment if I need to add some additional info.