tracker890 Source h
- 90
- 11
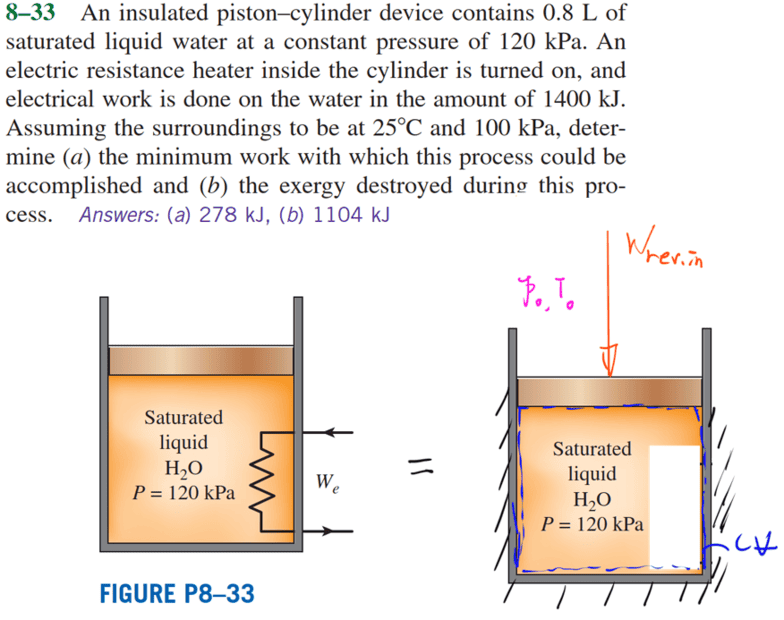
- Homework Statement
- Puzzled by why it's a reversible adiabatic process but not isentropic.
- Relevant Equations
- Wb,rev equation
Q1: Why can't set ##Q_{in,net}=0## and use equation (2) to obtain ##W_{act,in}=\left( \bigtriangleup U \right) _{cv}##?
Q2: If assume it's a reversible process, why can't equation (3) determine (△S)sys=0?
ref1.
ref2.
$$
W_{rev,in}=W_{act,in}-W_{surr}=W_{act,in}+P_0\left( V_2-V_1 \right) \cdots \text{(1)}
$$
$$
\ \ Q_{in,net}+W_{act,in}=\left( \bigtriangleup U \right) _{cv}\cdots \text{(2)}
$$
$$
\ \ T_0\left( \bigtriangleup S \right) _{sys}=\sum{\left( \frac{T_0}{T_k} \right)}Q_k\cdots \text{(3)}
$$
$$
\ let\ \ Q_{in,net}=Q_k
$$
$$
\left( 2 \right) +\left( 3 \right) :\
$$
$$
\ \ W_{act,in}=\sum{\left( -1+\frac{T_0}{T_k} \right)}\cancel{Q_k}+\left( \bigtriangleup U \right) _{cv}-\ T_0\left( \bigtriangleup S \right) _{sys}
$$
$$
\ \ \ \ \ \ \ \ =\left( U_2-U_1 \right) -T_0\left( S_2-S_1 \right) _{sys}\cdots \text{(4)}
$$
$$
Substitute\left( 4 \right) into\left( 1 \right)
$$
$$
W_{rev,in}=\left( U_2-U_1 \right) -T_0\left( S_2-S_1 \right) _{sys}+P_0\left( V_2-V_1 \right)
$$
$$
=m\left[ \left( u_2-u_1 \right) -T_0\left( s_2-s_1 \right) _{sys}+P_0\left( v_2-v_1 \right) \right]
$$
$$
=-m\left[ \left( u_1-u_2 \right) -T_0\left( s_1-s_2 \right) _{sys}+P_0\left( v_1-v_2 \right) \right] \cdots \text{(5)}
$$
Q2: If assume it's a reversible process, why can't equation (3) determine (△S)sys=0?
ref1.
ref2.
$$
W_{rev,in}=W_{act,in}-W_{surr}=W_{act,in}+P_0\left( V_2-V_1 \right) \cdots \text{(1)}
$$
$$
\ \ Q_{in,net}+W_{act,in}=\left( \bigtriangleup U \right) _{cv}\cdots \text{(2)}
$$
$$
\ \ T_0\left( \bigtriangleup S \right) _{sys}=\sum{\left( \frac{T_0}{T_k} \right)}Q_k\cdots \text{(3)}
$$
$$
\ let\ \ Q_{in,net}=Q_k
$$
$$
\left( 2 \right) +\left( 3 \right) :\
$$
$$
\ \ W_{act,in}=\sum{\left( -1+\frac{T_0}{T_k} \right)}\cancel{Q_k}+\left( \bigtriangleup U \right) _{cv}-\ T_0\left( \bigtriangleup S \right) _{sys}
$$
$$
\ \ \ \ \ \ \ \ =\left( U_2-U_1 \right) -T_0\left( S_2-S_1 \right) _{sys}\cdots \text{(4)}
$$
$$
Substitute\left( 4 \right) into\left( 1 \right)
$$
$$
W_{rev,in}=\left( U_2-U_1 \right) -T_0\left( S_2-S_1 \right) _{sys}+P_0\left( V_2-V_1 \right)
$$
$$
=m\left[ \left( u_2-u_1 \right) -T_0\left( s_2-s_1 \right) _{sys}+P_0\left( v_2-v_1 \right) \right]
$$
$$
=-m\left[ \left( u_1-u_2 \right) -T_0\left( s_1-s_2 \right) _{sys}+P_0\left( v_1-v_2 \right) \right] \cdots \text{(5)}
$$