- #1
- 2,138
- 2,713
My book gives the following graph for current vs accelerating potential for Franck and Hertz experiment (used to prove existence of discrete energy levels in atoms) using Mercury vapours in the tube:
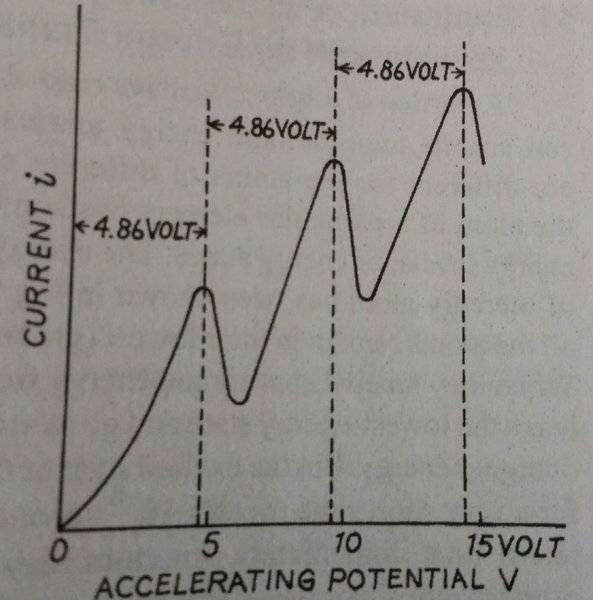
The book then writes:
"Actually, atoms have more than one excitation potential and also an ionisation potential. For example, the second excitation potential if Mercury is 6.67 volt and the ionisation potential is 10.4 volt. Hence, the second and third peaks in the graph become complicated."
Now, is this Ionisation Potential the same as "amount of energy required to remove the most loosely bound electron from the outermost shell of an isolated, neutral, gaseous atom to form a unicharged ion"? If so, then how is that measured? If not, then what is the proper definition?
Secondly, how will the graph change?
The book then writes:
"Actually, atoms have more than one excitation potential and also an ionisation potential. For example, the second excitation potential if Mercury is 6.67 volt and the ionisation potential is 10.4 volt. Hence, the second and third peaks in the graph become complicated."
Now, is this Ionisation Potential the same as "amount of energy required to remove the most loosely bound electron from the outermost shell of an isolated, neutral, gaseous atom to form a unicharged ion"? If so, then how is that measured? If not, then what is the proper definition?
Secondly, how will the graph change?