Est120
- 54
- 3
- TL;DR Summary
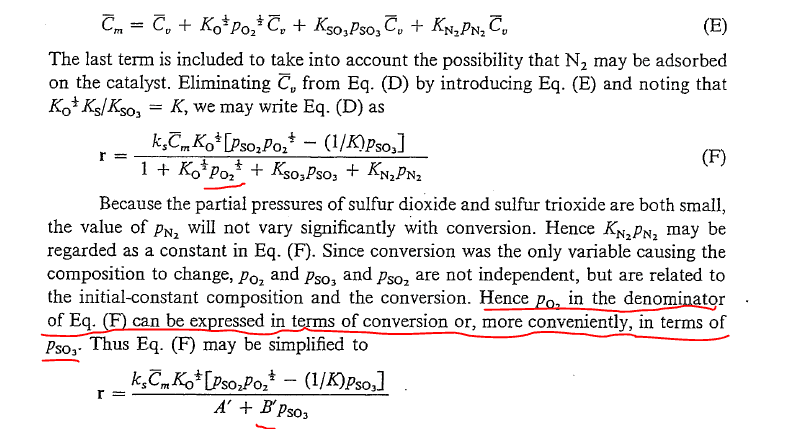
- reading J M Smith "Chemical engineering kinetics" there is a part in the book that i can't really understand
how does the partial pressure of oxygen (O2) that is raised to the (1/2) power "magically" combines to give PSO3? , i know that at constant Temperature you can pretty much express pressures between reactants and products using conversion but still you won't be able to factorize because they are raised to different exponent number, i think the author made an approximation but as usual they never explain anything, this book really sucks tbh lol