- #1
The Head
- 144
- 2
- TL;DR Summary
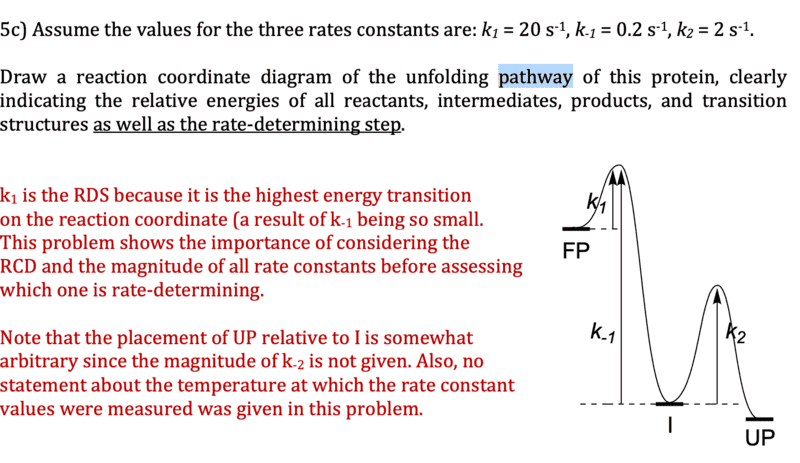
- How a k value can be the higher of the k values, but still an Rate-Determining Step
I'm trying to understand a little bit more about how k-values relate to rate-determining steps and energy diagrams. I always assumed that the lowest value of k (in the forward direction) was the RDS saw something in a handout that indicated otherwise. It explained that even though k1 > k2, because k-1 was so low, k1 would have the highest energy transition and that k1 was the RDS. I would have thought that would only indicate that k1 was irreversible, not the RDS.
Further, it showed an energy diagram that then displayed the activation energy being higher for k2, which thoroughly confused me, because I would think the one with the greater activation energy would be the RDS.
Can someone clarify for me what is going on? I'm including the image below for clarity's sake, but really just want to learn how to think about the principles at hand, so any help would be greatly appreciated!
Further, it showed an energy diagram that then displayed the activation energy being higher for k2, which thoroughly confused me, because I would think the one with the greater activation energy would be the RDS.
Can someone clarify for me what is going on? I'm including the image below for clarity's sake, but really just want to learn how to think about the principles at hand, so any help would be greatly appreciated!