- #1
engstud
- 1
- 0
Homework Statement
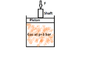
Figure P1.30 (attached) shows a gas contained in a vertical piston-cylinder assembly. A vertical shaft whose cross-sectional area is 0.8 cm2 is attached to the top of the piston. Determine the magnitude, F, of the force acting on the shaft, in N, required if the gas pressure is 3 bar. The masses of the piston and attached shaft are 24.5kg and 0.5kg respectively. The piston diameter is 10cm. The local atmospheric pressure is 1 bar. The piston moves smoothly in the cylinder and g=9.81 m/s2
Homework Equations
F=pA
F=ma
The Attempt at a Solution
I calculated the pressure exerted on the piston by the gas (I called it Fg) using Fg=pA which gave me Fg=(3 bar * 105 Pa)*pi*(.05m)^2=23561.9 N
I then drew a free body diagram of the piston, applied Newton's second law and ended up with 0=Fg-F-Ws-Wp where Ws is the weight of the shaft and Wp is the weight of the piston. When I plug in all my numbers and solve for F I end up with 23316.7N. I do not know whether or not this is the correct answer but I am being thrown off by the fact that they gave me the cross-sectional area of the shaft and I did not use it. Can someone please check my logic?