- #1
PainterGuy
- 940
- 70
Hi,
I was watching the following video. I need your help to clarify few important points. Thanks a lot in advance!
Question 1:
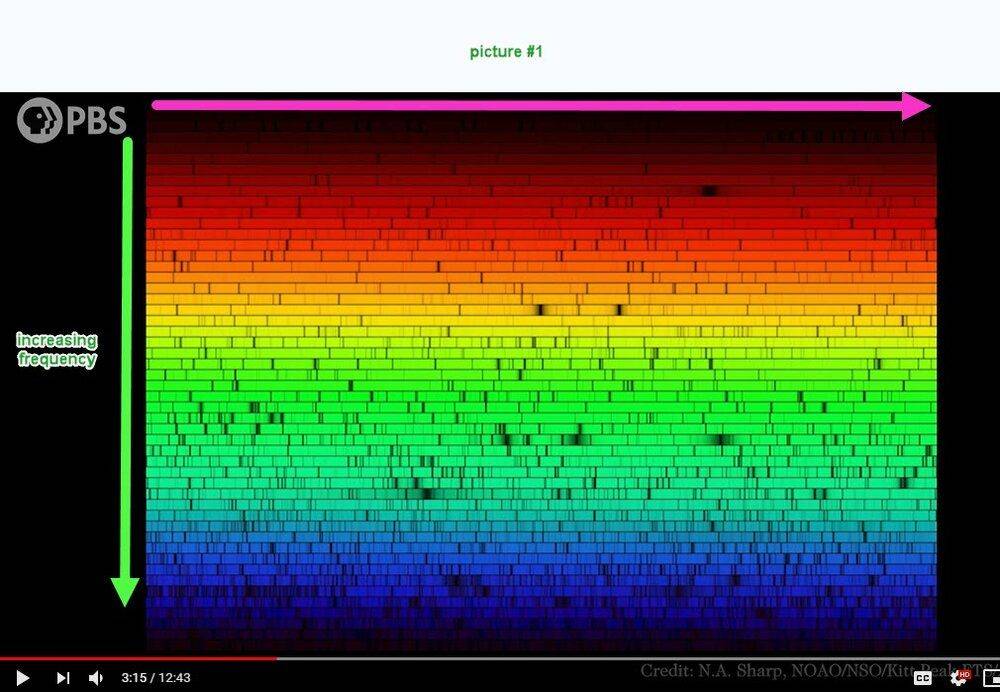
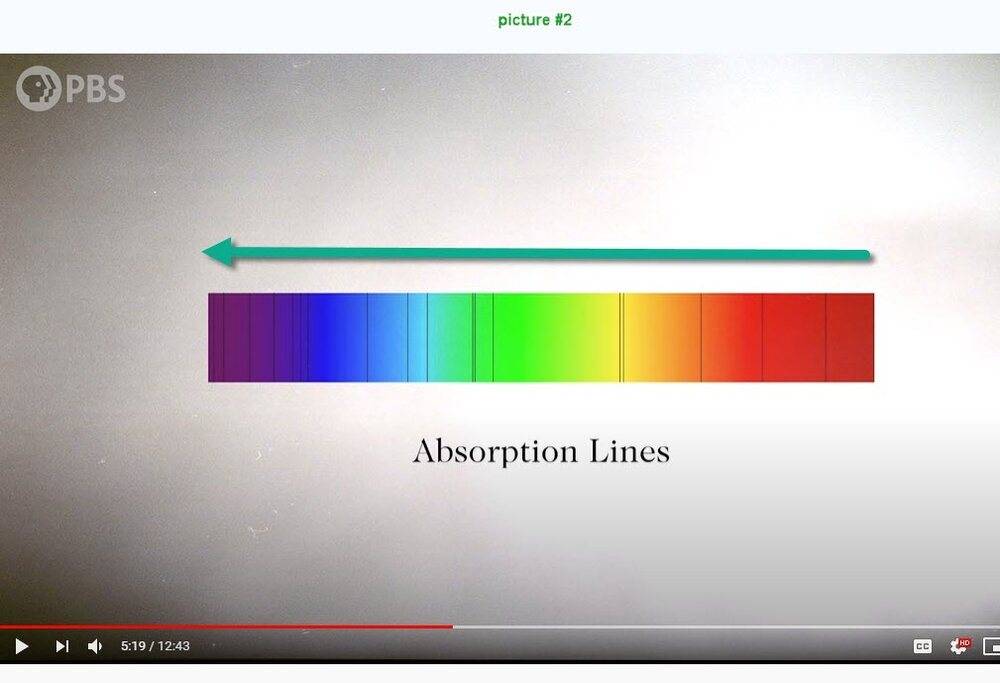
I'm not able to understand picture #1 below. I think that the frequency increases vertically downward but what about the horizontal scale? Compared to picture #1, picture #2 is quite understandable.
Question 2:
I don't think that dark lines represent total absence of certain frequencies rather the dark lines represent more of very low intensity frequencies compared to other brighter frequencies surrounding them. Do I have it correct?
Question 3:
Around 4:28 the following is said:
But wouldn't those atoms re-emit the photons after some time to get back to their ground state?
Question 4:
Don't atoms of elements also absorb photons of non-visible frequencies such as radio waves, infrared, ultraviolet, etc? As a layperson I have always seen visible spectrum being used when discussing absorption lines. Don't non-visible frequency photons also get absorbed?
I was watching the following video. I need your help to clarify few important points. Thanks a lot in advance!
Question 1:
I'm not able to understand picture #1 below. I think that the frequency increases vertically downward but what about the horizontal scale? Compared to picture #1, picture #2 is quite understandable.
Question 2:
I don't think that dark lines represent total absence of certain frequencies rather the dark lines represent more of very low intensity frequencies compared to other brighter frequencies surrounding them. Do I have it correct?
Question 3:
Around 4:28 the following is said:
By the time a photon reaches the photosphere it has a 50-50 chance of traveling the final 100km to space without bumping into anything. At least, that’s true for most of the light. But some photons encounter a new obstacle. As temperature drops, it becomes possible for some electrons to be captured by nuclei to form atoms. And if free electrons are good at stopping photons in their tracks, these atoms are even better. An atom can absorb a photon if doing so would cause one of its electrons to jump up to a higher energy level. The energy of the photon and the energy of the electron jump have to be exactly the same. So any photons trying to escape the Sun that happen to have one of these particular energies are going to get sucked up on its way out. And that’s what these dark lines are - we call them absorption lines. Each element on the periodic table produces a different set of lines corresponding to its unique energy levels. Just seeing which absorption lines are present tells you which elements are present inside the Sun.
But wouldn't those atoms re-emit the photons after some time to get back to their ground state?
Question 4:
Don't atoms of elements also absorb photons of non-visible frequencies such as radio waves, infrared, ultraviolet, etc? As a layperson I have always seen visible spectrum being used when discussing absorption lines. Don't non-visible frequency photons also get absorbed?