samy4408
- 62
- 9
Hello , the problem that i refer to is present when we try to calculate the pH of a solution without any assumption.
in a lecture entitled "calculating the pH of a strong acid/base solution " after adding a certain amount of HCl in water we are asked to calculate the pH
(the amount of HCl and volume of water is known )
*to calculate the pH we need to calculate [H3O+]
*we have 2 sources of [H3O+] in this solution
-first the [H3O+] from HCl
-second the [H3O+] from the dessosiation of water
we know that [H3O+]from HCl=[Cl-] because HCl is a strong acid
and we reffered to the [H3O+] from water as [OH-] ( here is the problem but we'll come back to it later )
using Kw we replaced [OH-] with (10^-14/[H3O+])
now we have this equation [H3O+]=(10^-14/[H3O+]) +[Cl-]
and the professor said that all we need to do is to solve the quadratic equation
here is the problem it seem that the two [H3O+] are not the same unknown ( the amount of [H3O+] from water do not equal to (10^-14/[H3O+])) , here is a plot
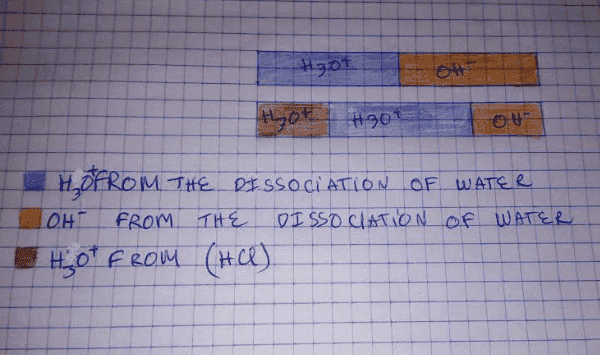
in a lecture entitled "calculating the pH of a strong acid/base solution " after adding a certain amount of HCl in water we are asked to calculate the pH
(the amount of HCl and volume of water is known )
*to calculate the pH we need to calculate [H3O+]
*we have 2 sources of [H3O+] in this solution
-first the [H3O+] from HCl
-second the [H3O+] from the dessosiation of water
we know that [H3O+]from HCl=[Cl-] because HCl is a strong acid
and we reffered to the [H3O+] from water as [OH-] ( here is the problem but we'll come back to it later )
using Kw we replaced [OH-] with (10^-14/[H3O+])
now we have this equation [H3O+]=(10^-14/[H3O+]) +[Cl-]
and the professor said that all we need to do is to solve the quadratic equation
here is the problem it seem that the two [H3O+] are not the same unknown ( the amount of [H3O+] from water do not equal to (10^-14/[H3O+])) , here is a plot