Viona
- 49
- 12
- Homework Statement
- Why we calculate the magnetic field of the proton (B) using the angular momentum of the electron (L)?
- Relevant Equations
- Why we had to do this calculations in the rest frame
of the electron?
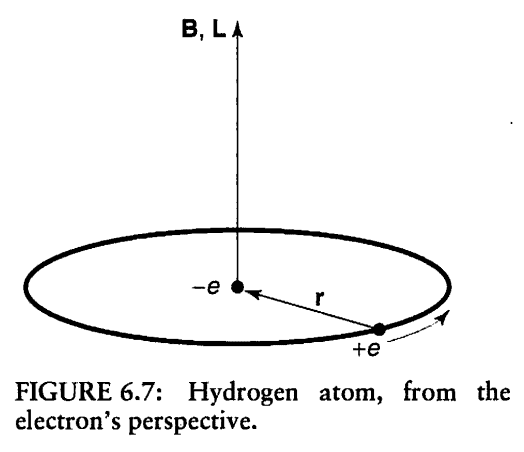
I was reading in the Book: Introduction to Quantum Mechanics by David J. Griffiths. In chapter Time-independent Perturbation Theory, Section: Spin -Orbit Coupling. I understood that the spin–orbit coupling in Hydrogen atom arises from the interaction between the electron’s spin magnetic moment, and the proton’s orbital magnetic field B. But I did not understand why He calculated the magnetic field of the proton (B) using the angular momentum of the electron (L)! Please see the attached pictures. Thank you!