- #1
Spacetime walker
- 4
- 0
- TL;DR Summary
- A question about the possibility of separating a one-particle wave function by infinitely high potential walls.
Hello all, I am a newcomer here. Not a physicist, just an enthusiast. ;)
I was thinking whether it is possible to separate a one-particle wave function into two, "completely disjoint" parts. The following thought experiment explains better what I am thinking about.
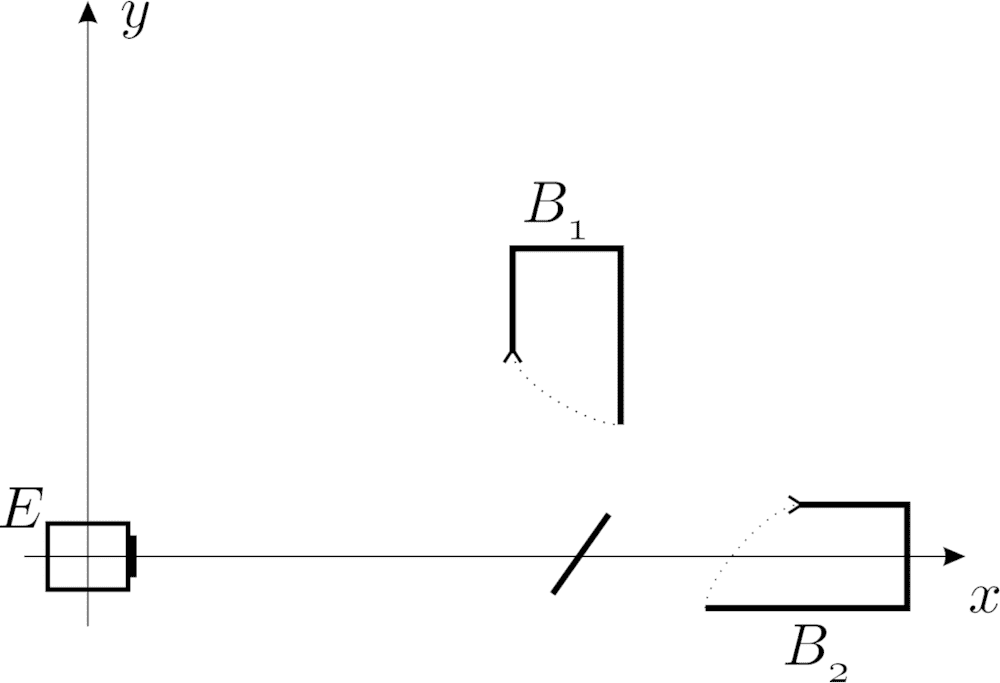
Let us suppose, that there is an emitter E in the origin of our coordinate system (see the below figure), and it emits an electron such that this electron moves in the x direction to the right (more realistically, its momentum points to the positive x direction with very high probability).
In some point on the right of the origin, there is a semi-transparent mirror rotated by 45 degrees. After the electron reaches this mirror, the wave function will have two peaks and these start to evolve in both directions (upwards, and to the right).
Suppose also that we have put a box in both of the possible electron-directions at some but equal distances from the mirror (B_1 and B_2 in the Figure). These boxes automatically close shortly after the electron is supposed to reach there (for this arrival time, you do not need to observe the electrons, it is enough to know the characteristics of the emitter, I think).
Thus, the boxes (like infinitely high potential walls) trap the wave function peaks. At the instance when the doors close, the wave function will completely be split into two parts without physical interaction possible. But still, we are talking about one particle.
My questions:
1) What I have written above was dictated by my intuition. Is this complete separation possible by the principles of QM?
2) And if it is possible, how can you explain the "spooky action at a distance" when you open one of the boxes at a later time if the box walls do not permit "communication" between the parts? (The wave function in the non-opened box must change instantaneously as well.)
Thank you for your answers, in advance! ;)
I was thinking whether it is possible to separate a one-particle wave function into two, "completely disjoint" parts. The following thought experiment explains better what I am thinking about.
Let us suppose, that there is an emitter E in the origin of our coordinate system (see the below figure), and it emits an electron such that this electron moves in the x direction to the right (more realistically, its momentum points to the positive x direction with very high probability).
In some point on the right of the origin, there is a semi-transparent mirror rotated by 45 degrees. After the electron reaches this mirror, the wave function will have two peaks and these start to evolve in both directions (upwards, and to the right).
Suppose also that we have put a box in both of the possible electron-directions at some but equal distances from the mirror (B_1 and B_2 in the Figure). These boxes automatically close shortly after the electron is supposed to reach there (for this arrival time, you do not need to observe the electrons, it is enough to know the characteristics of the emitter, I think).
Thus, the boxes (like infinitely high potential walls) trap the wave function peaks. At the instance when the doors close, the wave function will completely be split into two parts without physical interaction possible. But still, we are talking about one particle.
My questions:
1) What I have written above was dictated by my intuition. Is this complete separation possible by the principles of QM?
2) And if it is possible, how can you explain the "spooky action at a distance" when you open one of the boxes at a later time if the box walls do not permit "communication" between the parts? (The wave function in the non-opened box must change instantaneously as well.)
Thank you for your answers, in advance! ;)