- #1
crays
- 160
- 0
Hi guys, here i have two question, but i don't understand the answer at all.
For the standard enthalphy change question 5) b) i) shouldn't it be standard enthalphy change = Product - Reactant? But from the answer it was given as reactant - product.
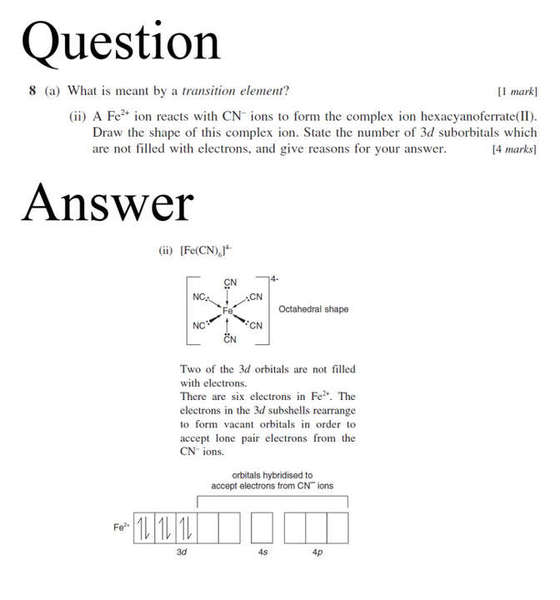
For question number 8, i don't understand why Fe2+ still has 6 valence electrons, shouldn't it be 4?

For the standard enthalphy change question 5) b) i) shouldn't it be standard enthalphy change = Product - Reactant? But from the answer it was given as reactant - product.
For question number 8, i don't understand why Fe2+ still has 6 valence electrons, shouldn't it be 4?