- #1
Decimal
- 75
- 7
Hello,
I have a question regarding the derivation for Bose Einstein condensation. I understand that in a boson gas for high temperatures the expectation value of the total number of particles should equal something like: $$ \langle N \rangle \sim T * \eta(z)$$ With ## z = exp(\frac {\mu} {k_b T})##, ##T## the temperature and $$ \eta(z) = \int_0^\infty \frac{x^2}{z^{-1}*exp(x)-1} \, dx$$ This function ##\eta(z)## increases for decreasing ##z## until it reaches a maximum at ##z = 1##. The graph looks like this:
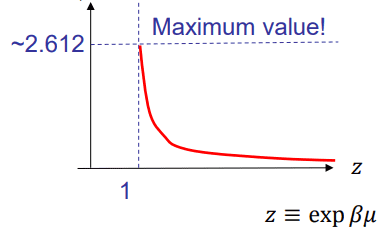
Now if I understand correctly, what you want to do is start cooling the system whilst keeping the number of particles constant. My question is, how? If you decrease ##T## then ##z## will increase thus lowering the value of ##\eta(z)##. Because of this the number of particles should always decrease right? I feel like I am missing something here so maybe someone could explain?
Thanks!
Edit: So I feel like I should clarify a couple of things. The above expression for ## \langle N \rangle## gives the number of particles in the excited state. In other words, all particles except the ones in the ground state. Now my lecture notes explain that one can lower the temperature whilst keeping the number of particles (in excited states) constant, up until the ##\eta(z)## maxes out at ##z=1##. After this point particles will start to move to the ground state which defines the critical temperature. I understand this, but still don't see how the number of particles can be held constant at all. Lowering ##T## also lowers ##\eta(z)## so number of particles will never be held constant right? Lowering the temperature immediately lowers the number of particles in the excited states.
I have a question regarding the derivation for Bose Einstein condensation. I understand that in a boson gas for high temperatures the expectation value of the total number of particles should equal something like: $$ \langle N \rangle \sim T * \eta(z)$$ With ## z = exp(\frac {\mu} {k_b T})##, ##T## the temperature and $$ \eta(z) = \int_0^\infty \frac{x^2}{z^{-1}*exp(x)-1} \, dx$$ This function ##\eta(z)## increases for decreasing ##z## until it reaches a maximum at ##z = 1##. The graph looks like this:
Now if I understand correctly, what you want to do is start cooling the system whilst keeping the number of particles constant. My question is, how? If you decrease ##T## then ##z## will increase thus lowering the value of ##\eta(z)##. Because of this the number of particles should always decrease right? I feel like I am missing something here so maybe someone could explain?
Thanks!
Edit: So I feel like I should clarify a couple of things. The above expression for ## \langle N \rangle## gives the number of particles in the excited state. In other words, all particles except the ones in the ground state. Now my lecture notes explain that one can lower the temperature whilst keeping the number of particles (in excited states) constant, up until the ##\eta(z)## maxes out at ##z=1##. After this point particles will start to move to the ground state which defines the critical temperature. I understand this, but still don't see how the number of particles can be held constant at all. Lowering ##T## also lowers ##\eta(z)## so number of particles will never be held constant right? Lowering the temperature immediately lowers the number of particles in the excited states.
Attachments
Last edited:
