hokhani
- 561
- 18
- TL;DR Summary
- Understanding the relation between system symmetry and wave-function symmetry
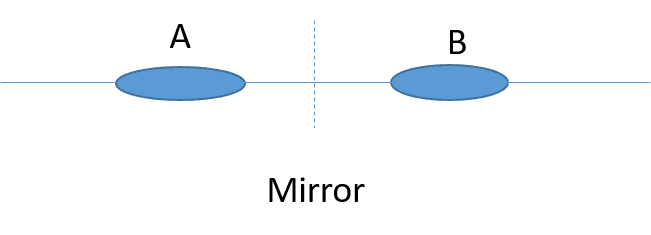
In the picture below we have two identical orbitals A and B and the system has left-right symmetry. I use the notation ##|n_{A \uparrow}, n_{A \downarrow},n_{B \uparrow},n_{B \downarrow}>## which for example ##n_{A \uparrow}## indicates the number of spin-up electrons in the orbital A. I would like to know is it possible to have an eigenstate as ##|1,1,0,0>## in this left-right symmetric system or, because of the symmetry of system, we must only have symmetric wave-functions as ##\frac{|1,1,0,0>\pm|0,0,1,1>}{\sqrt(2)}##?
Any help is appreciated.
Any help is appreciated.