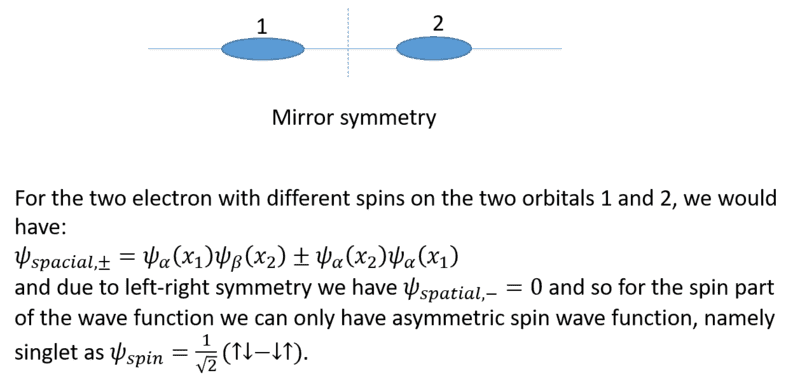
What we are discussing is best described by a tensor product of the form ##\text{spatial part} \otimes \text{spin part}##, which can be done in non-relativistic approximation, where the spin commutes with position and momentum operators. For a system of two spin-1/2 particles as discussed here, there are two possibilities:
$$\Psi(\vec{x}_1,\sigma_1;\vec{x}_2 \sigma_2)=\psi_{\text{even}}(\vec{x}_1,\vec{x}_2) \otimes \chi^{(S=0)}(\sigma_1,\sigma_2)$$
or
$$\Psi(\vec{x}_1,\sigma_2;x_2 \sigma_2) = \psi_{\text{odd}}(\vec{x}_1,\vec{x}_2) \otimes \chi^{(S=1)}(\sigma_1,\sigma_2).$$
In the first case there's only one spin state,
$$\chi^{(S=0)}(\sigma_1,\sigma_2)=\frac{1}{\sqrt{2}} (|1/2,-1/2 \rangle - |-1/2,1/2 \rangle),$$
while in the 2nd case
$$\chi^{(S=1})(\sigma_1,\sigma_2) = \lambda_1 |1/2,1/2 \rangle + \lambda_0 \frac{1}{\sqrt{2}} (|1/2,-1/2 \rangle + |-1/2,1/2 \rangle_ + \lambda_{-1} |-1/2,-1/2 \rangle.$$
Since we must have
$$\Psi(\vec{x}_1,\sigma_1;\vec{x}_2,\sigma_2)=-\Psi(\vec{x}_2,\sigma_2;\vec{x}_1,\sigma_1),$$
obviously we must have
$$\psi_{\text{even}}(\vec{x}_1,\vec{x}_2)=\psi_{\text{even}}(\vec{x}_2,\vec{x}_1), \quad \psi_{\text{odd}}(\vec{x}_1,\vec{x}_2)=-\psi_{\text{odd}}(\vec{x}_2,\vec{x}_1),$$
because
$$\chi^{(S=0)}(\sigma_1,\sigma_2)=-\chi^{(S=0)}(\sigma_2,\sigma_1), \quad \chi^{(S=1)}(\sigma_1,\sigma_2) = \chi^{(S=1)}(\sigma_2,\sigma_1).$$