- #1
SpectraPhy09
- 25
- 3
- Homework Statement
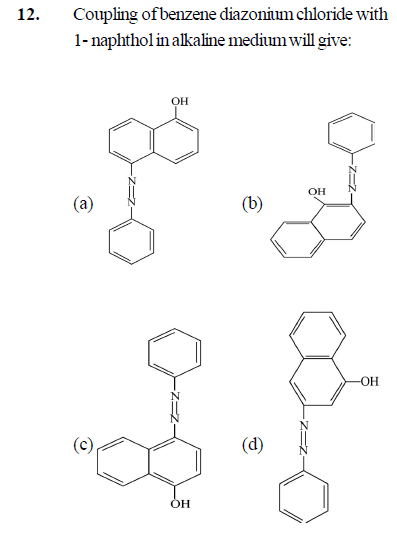
- Coupling of the benzene diazonium chloride with the 1-Naphthol in the alkaline medium will give: (For options plz check the image I have attached )
- Relevant Equations
- I don't know any
I think that since the -Oh group is attached it would be activating the ring so either ortho product will be formed or the para product. So I'm confused between option b & c (Given and in my textbook is C).
Plz can someone help me with this
Plz can someone help me with this
Last edited by a moderator: