mohamed_a
- 36
- 6
I was reading about thermodynamics in my textbook wheni came across the following thermodynamics constants:
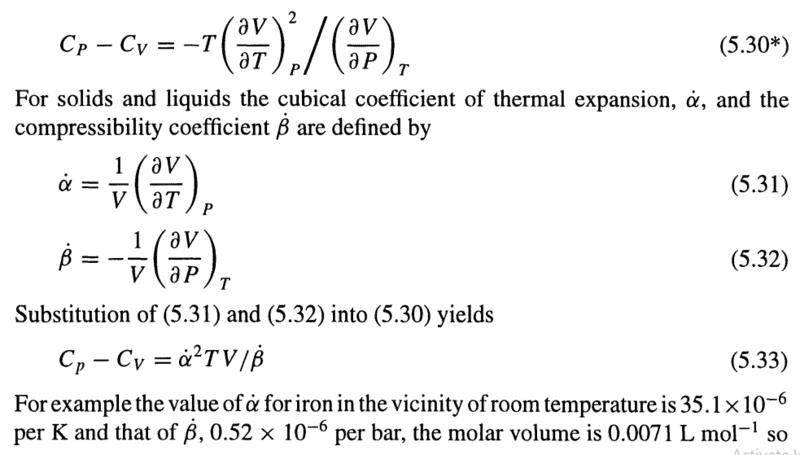
However, i don't understand why did we define 1/V inthe constants. What is the point in doing this?
However, i don't understand why did we define 1/V inthe constants. What is the point in doing this?