- #1
Vitalius6189
- 9
- 1
Homework Statement
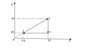
A mass of gas occupying volume V1 = 2 m3 at the pressure P1 = 4*10^5 Pa performs the cycle represented in the Figure that i have uploaded.What is the work of gas in this cycle, knowing that the pressure P2 = 10^5 Pa

Homework Equations
Work=1/2 * (P1 - P2) * (V1 - V3)
The Attempt at a Solution
The work is the area within the triangle.
1/2 base * height
= 1/2 * (P1 - P2) * (V1 - V3) The question that i have is how do i find V3?[/B]