- #1
WrongMan
- 149
- 15
Homework Statement
a system uses monoatomic ideal gas describing the following cycle (quasi static)
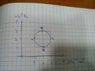
and basically the p-v graph is a circle,and it starts on the left side of the circle with states A, B, C, D (so that B and D are top an bottom) and so P on A (PA) is equal to PC and VB= VD
given values:
Cv=3/2R
PA=PC= 2*105Pa
PD=1*105Pa
PB=3*105Pa
VA=0.001m3
VB=VD=0.002m3
VC=0.003m3.
what is the work done by the gas?
The Attempt at a Solution
so iv realized B to C and D to A are isothermal and i can calculate work for those, but the other two arent and they also arent adiabatic.
Im lost. there are so many relations due the first law, i don't know what to do. please a hint.