Graham87
- 72
- 16
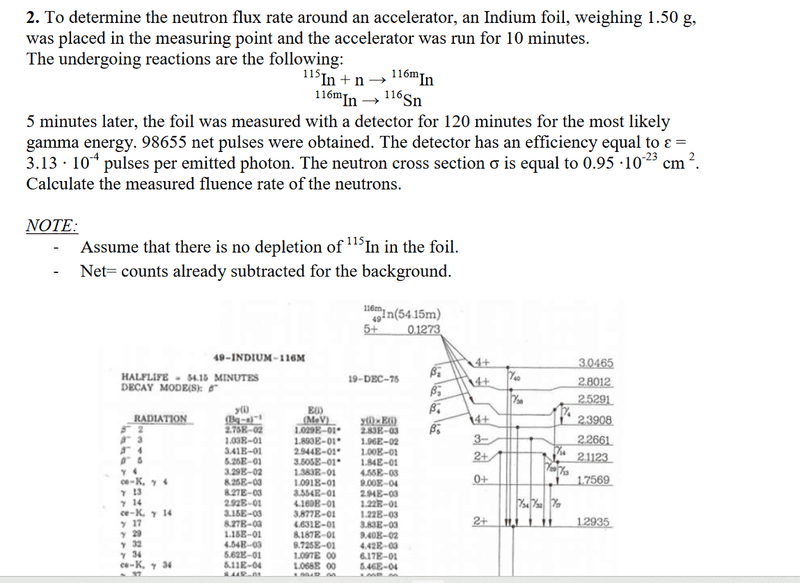
- Homework Statement
- See pictures
- Relevant Equations
- See pictures
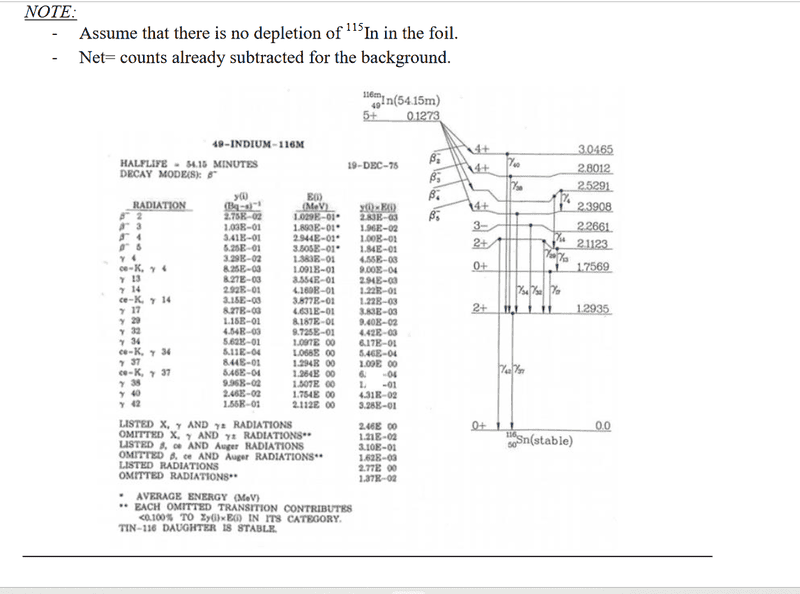
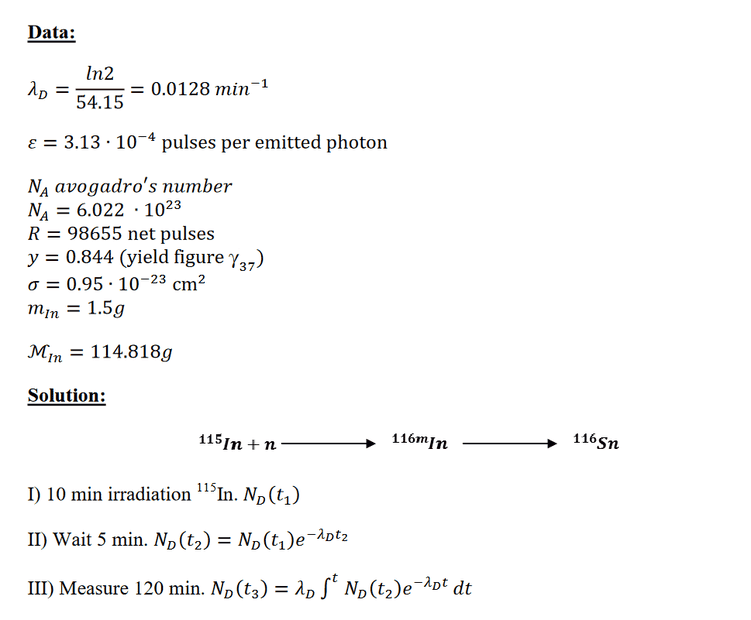
The problem comes with solutions. However, I dont get the 3 steps in the solutions. Why do they calculate decay for 120min in step 3? And why is only the daughter nuclide relevant and no granddaughter? There might be something lacking in my knowledge about nuclear reactions.
Also, I don't know what to use with the information "most likely gamma ray" in the question. I know it is gamma37, but what should I derive from knowing that?
Thanks!
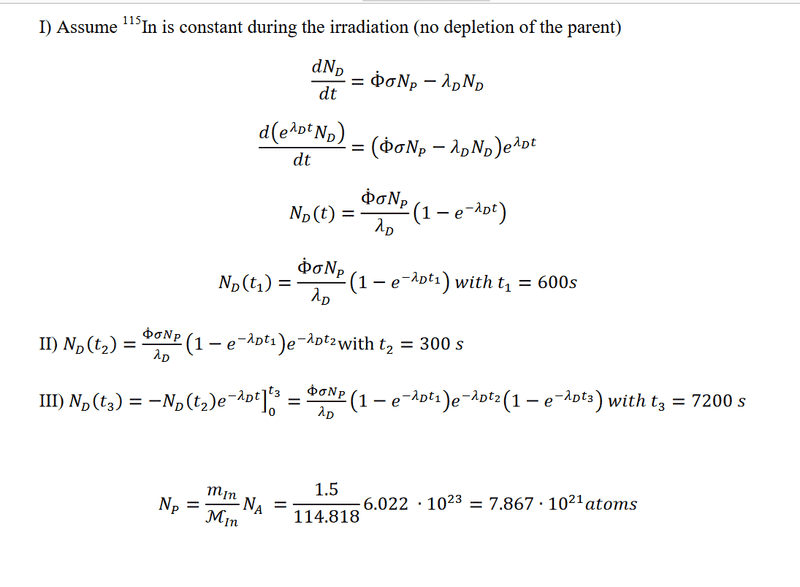
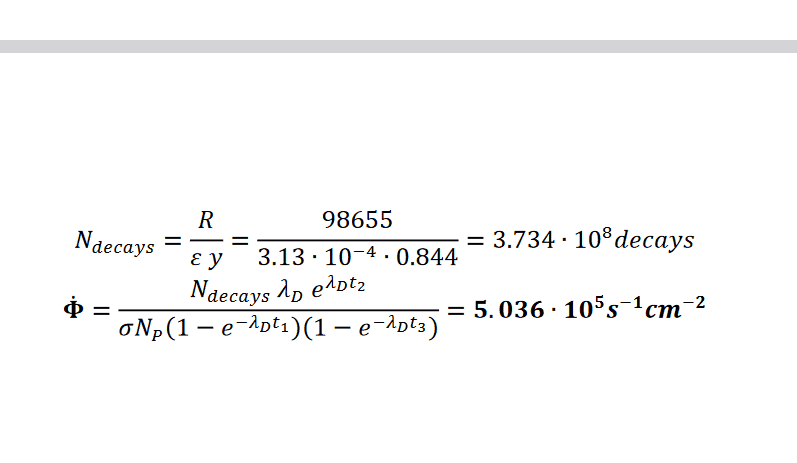
Also, I don't know what to use with the information "most likely gamma ray" in the question. I know it is gamma37, but what should I derive from knowing that?
Thanks!
Last edited by a moderator: