Albertgauss
Gold Member
- 294
- 37
TL;DR Summary: I have a Force Table Lab and am not sure if the "percent error" is the correct way to express errors. I also need some help on sig figs for my results.
Hello,
I am working with the usual undergrad force tables apparatus. I would like to know how to express the results of measurements and calculations properly for such an apparatus and make the measurements to the correct number of significant figures.
Here is a picture of the situation to show the beginning of the problem:
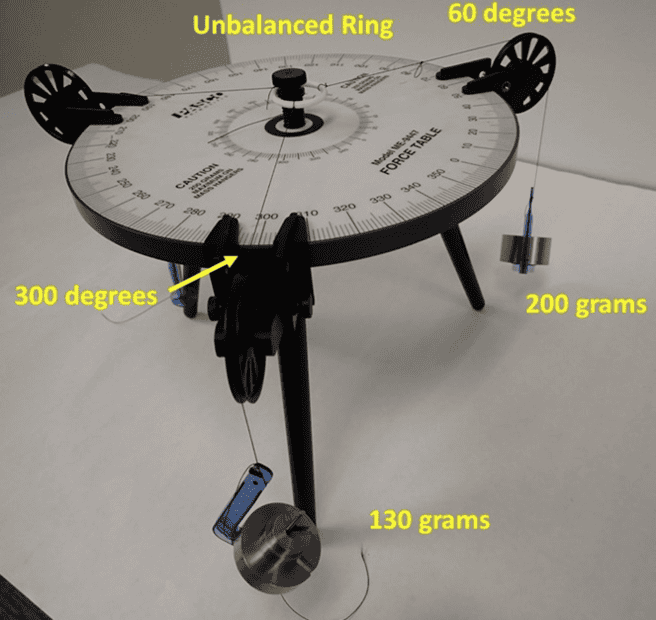
The ring is unbalanced by two masses, a 130 gram mass at 300 degrees and a 200 gram mass at 60 degrees. I calculate that I would need (using the usual force vector math) to hang a 175.78 gram mass at 200.17 degrees to balance the ring (such that it floats and does not touch the center peg). These are my theory values.
Here is a schematic of the situation.
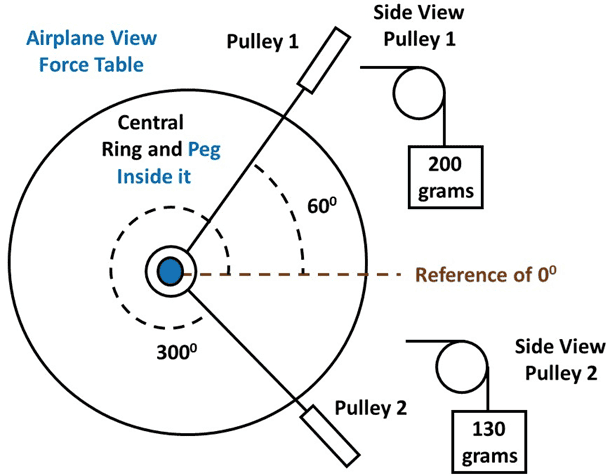
When I did the actual experiment, I measured that the mass to balance my system should be 180 grams at 194 degrees, and you can see that below.
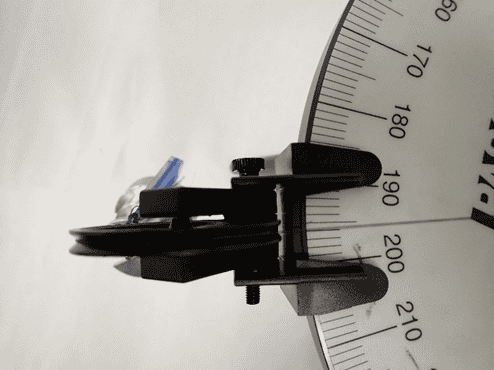
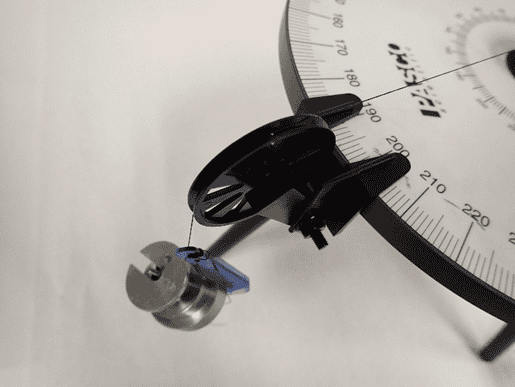
In the left figure you can see that I can measure the angle no better reliably than to one degree; a finer increment than one degree on the force table and I can’t really measure that. In the right-hand side, those masses lumped together total 180 grams. I could put on little disks of one gram or so, but no masses smaller than that.
How do I properly express my measurements in terms of my limiting ability to measure the angle at no better than one degree or my mass to no better than one gram? I do have a percent error formula I can work with and it goes as follows:
100*Absolute Value of (theory value subtract experimentally measured value)/(theory value)
The percent error on the mass is 2.4% and the percent error on the angle direction is 2.6%. Would this suffice for error analysis or is there a better way to do it? How do I report these findings with correct significant figures?
Hello,
I am working with the usual undergrad force tables apparatus. I would like to know how to express the results of measurements and calculations properly for such an apparatus and make the measurements to the correct number of significant figures.
Here is a picture of the situation to show the beginning of the problem:
The ring is unbalanced by two masses, a 130 gram mass at 300 degrees and a 200 gram mass at 60 degrees. I calculate that I would need (using the usual force vector math) to hang a 175.78 gram mass at 200.17 degrees to balance the ring (such that it floats and does not touch the center peg). These are my theory values.
Here is a schematic of the situation.
When I did the actual experiment, I measured that the mass to balance my system should be 180 grams at 194 degrees, and you can see that below.
In the left figure you can see that I can measure the angle no better reliably than to one degree; a finer increment than one degree on the force table and I can’t really measure that. In the right-hand side, those masses lumped together total 180 grams. I could put on little disks of one gram or so, but no masses smaller than that.
How do I properly express my measurements in terms of my limiting ability to measure the angle at no better than one degree or my mass to no better than one gram? I do have a percent error formula I can work with and it goes as follows:
100*Absolute Value of (theory value subtract experimentally measured value)/(theory value)
The percent error on the mass is 2.4% and the percent error on the angle direction is 2.6%. Would this suffice for error analysis or is there a better way to do it? How do I report these findings with correct significant figures?