pisluca99
- 63
- 4
- TL;DR Summary
- Gibbs Free Energy Graphs
Hi everybody,
I don't understand what changes between these two graphs. In particular, why does free energy reach a minimum in one graph and a maximum in the other? Shouldn't a chemical reaction always have an energy maximum, represented by the activation energy?
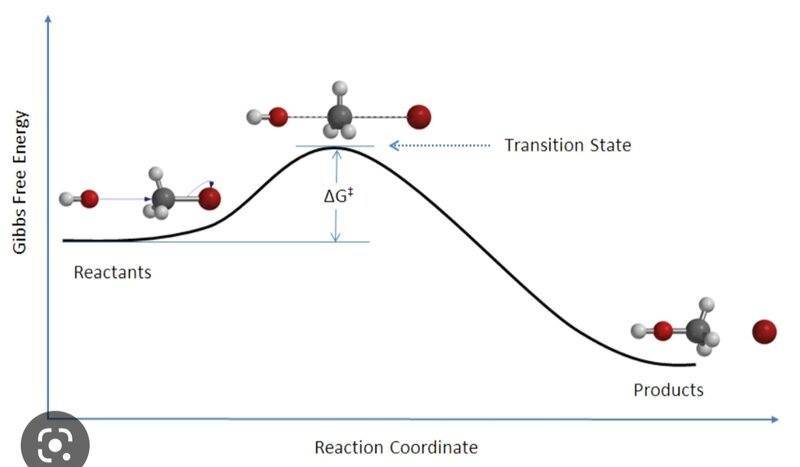
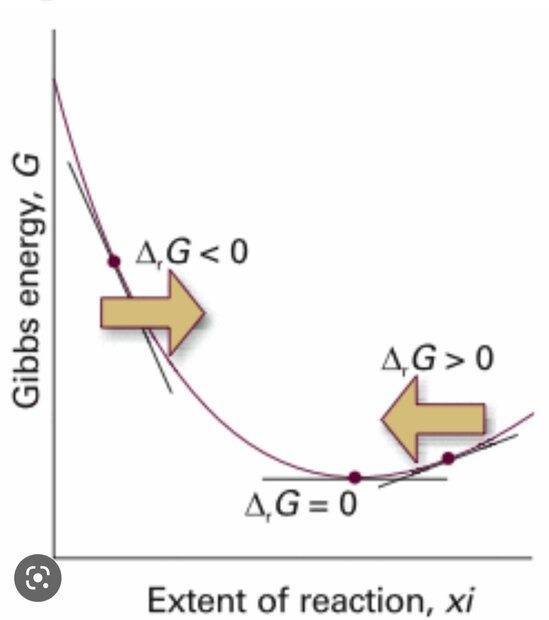
I don't understand what changes between these two graphs. In particular, why does free energy reach a minimum in one graph and a maximum in the other? Shouldn't a chemical reaction always have an energy maximum, represented by the activation energy?