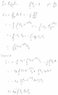- #1
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Using the partition function find the entropy
- Thread starter ber70
- Start date
In summary, the partition function is a mathematical function used in statistical mechanics to calculate the thermodynamic properties of a system. It is used to find the entropy of a system by using the formula S = k<sub>B</sub> ln(Z). The partition function is directly related to entropy, indicating a greater amount of disorder in the system. It can be used to find the entropy of any physical system, as long as the energy levels and degeneracy of the particles are known. Temperature has a direct impact on the partition function and entropy, with increasing temperature leading to a higher partition function and entropy due to increased particle energy and disorder in the system.
FAQ: Using the partition function find the entropy
What is the partition function?
The partition function is a mathematical function used in statistical mechanics to calculate the thermodynamic properties of a system. It takes into account the energy levels and degeneracy of the particles in a system.
How is the partition function used to find entropy?
The partition function is used to find the entropy of a system by using the formula S = kB ln(Z), where S is the entropy, kB is the Boltzmann constant, and Z is the partition function.
What is the relationship between partition function and entropy?
The partition function is directly related to entropy, as it is used to calculate the entropy of a system. The higher the partition function, the higher the entropy, indicating a greater amount of disorder in the system.
Can the partition function be used to find the entropy of any system?
Yes, the partition function can be used to find the entropy of any physical system, as long as the energy levels and degeneracy of the particles in the system are known.
How does temperature affect the partition function and entropy?
Temperature has a direct impact on the partition function and entropy. As temperature increases, the partition function increases, leading to a higher entropy. This is because at higher temperatures, particles have more energy and are therefore more likely to occupy higher energy levels, increasing the overall disorder of the system.
Similar threads
- Replies
- 1
- Views
- 1K
- Replies
- 1
- Views
- 701
- Replies
- 9
- Views
- 4K
- Replies
- 1
- Views
- 2K
- Replies
- 5
- Views
- 2K
- Replies
- 1
- Views
- 929
- Replies
- 1
- Views
- 1K
- Replies
- 3
- Views
- 2K
- Replies
- 2
- Views
- 2K
- Replies
- 1
- Views
- 2K
Share: