StarChem
- 6
- 0
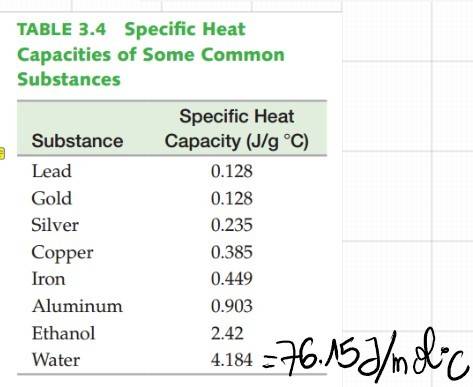
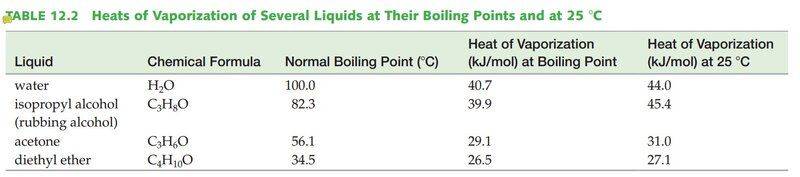
According to the Vaporization Heat table, the heat needed for 1 mol of H2O to evaporate at 100°C is 40.7KJ and 44.0KJ/mol is needed to evaporate H2O at 25°C. Thus 44.0-40.7=3.7KJ is the energy needed to heat H2O to 100°C from 25°C. However, according to the heat capacity of H2O, 3.7KJ will only warm the water by ~+48.6°C, which is not enough to reach 100°C starting from 25°C!
Last edited by a moderator: