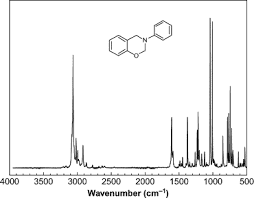
To add to what has already been said, an important distinction to make is the x-axis in wavenumbers is typically labeled as Raman Shift. This shift is relative to the Rayleigh scattering line at 0 cm
-1 and corresponds to the process by which the scattered light being measured is at the same frequency as the incident light source.
When a peak appears in a Raman spectrum, what is being measured is the frequency of a molecular vibration that is being driven by the incident light. You can think of this process as excitation from the ground state to some excited (virtual) state followed by de-excitation to a low-energy vibrational state with a corresponding emission of a photon. This emitted photon will be slightly different in energy (or frequency since E = hf) and this difference in the frequency of the incident photon and the frequency of the outgoing photon is what produces Raman peaks. The molecule is left in an excited vibrational state and we can directly measure the frequency (energy) of this vibrational mode.
You can go a lot deeper with this type of measurement in conjunction with group theory analysis and/or DFT calculations, but for simpler molecules, a database can typically tell you what each peak represents. For example, peaks that appear around 3000 cm-1 are typically related to C-H vibrations, so you can look at the Raman spectra of a given molecule and start to piece together what each vibration physically looks like.
As for line-shape and broadening, what TeethWhitener said is correct - it is not always immediately obvious what causes broadening (or narrowing) of vibrational modes, but there are typically many things at play. For example, researchers typically can get better resolution in Raman spectra by having the sample to be measured in a fridge or cooled to near liquid nitrogen temperatures: typically, the cooler the sample, the narrower the peak. Also, it is worth noting that it is very likely that there are just a large number of vibrational modes that are closely spaced and that is why the peaks don't look like pure Lorentzians. As a rule of thumb, there will typically be 3N-6 vibrational modes for a given molecule where N is the number of atoms in the molecule. There is a lot more nuance to this as not all of those modes will be Raman active (based on selection rules), but you can already see that for a decent-sized molecule, there is potentially a very large number of vibrational modes that you can measure.