- #1
PainterGuy
- 940
- 70
Hi,
Could you please help me with the queries below?
Question 1:
Please have a look on the attachment, conduction_band2, or check the following link for better resolution http://imagizer.imageshack.us/a/img921/4356/vODcjh.jpg
It says, "Figure 1–7 shows energy diagrams for insulators, semiconductors, and conductors. The energy gap or band gap is the difference between two energy levels and is “not allowed” in quantum theory."
What does the part "is not allowed in quantum theory" really mean here? I don't think it means that in quantum theory band gap is not allowed. If it does mean this then why does the gap exist?
Question 2:
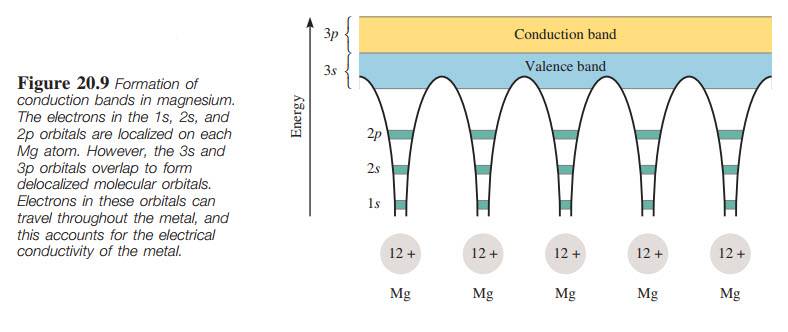
Please have a look on the attachment, conduction_band1, or check the following link for better resolution http://imagizer.imageshack.us/a/img924/8813/KsYTN0.jpg
What do these 'arches' mean? Are they really needed? Why would the author use this style?
Thank you for the help!
Could you please help me with the queries below?
Question 1:
Please have a look on the attachment, conduction_band2, or check the following link for better resolution http://imagizer.imageshack.us/a/img921/4356/vODcjh.jpg
It says, "Figure 1–7 shows energy diagrams for insulators, semiconductors, and conductors. The energy gap or band gap is the difference between two energy levels and is “not allowed” in quantum theory."
What does the part "is not allowed in quantum theory" really mean here? I don't think it means that in quantum theory band gap is not allowed. If it does mean this then why does the gap exist?
Question 2:
Please have a look on the attachment, conduction_band1, or check the following link for better resolution http://imagizer.imageshack.us/a/img924/8813/KsYTN0.jpg
What do these 'arches' mean? Are they really needed? Why would the author use this style?
Thank you for the help!

